SML2121
A-33
≥98% (HPLC)
同義詞:
2-(4-{[2-(5-Chlorothiophen-2-yl)-5-ethyl-6-methylpyrimidin-4-yl]amino}phenyl)acetic acid, 2-[4-[[2-(5-Chloro-2-thienyl)-5-ethyl-6-methyl-pyrimidin-4-yl]amino]phenyl]acetic acid, 4-[[2-(5-Chloro-2-thienyl)-5-ethyl-6-methyl-4-pyrimidinyl]amino]-benzeneacetic acid, A33
登入查看組織和合約定價
全部照片(1)
About This Item
推薦產品
化驗
≥98% (HPLC)
形狀
powder
顏色
white to beige
溶解度
DMSO: 2 mg/mL, clear
儲存溫度
2-8°C
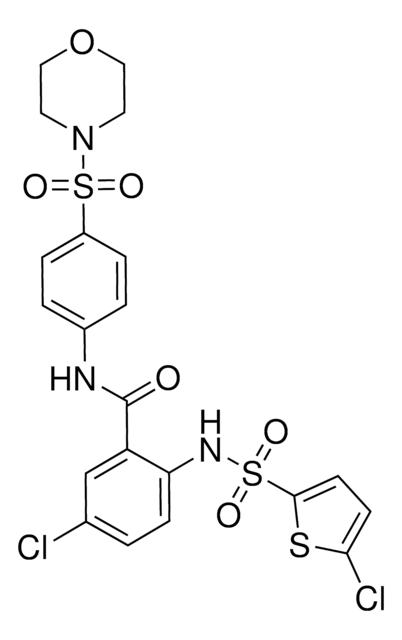
SMILES 字串
O=C(O)CC1=CC=C(NC2=NC(C3=CC=C(Cl)S3)=NC(C)=C2CC)C=C1
生化/生理作用
A-33 (A33) is a potent and selective catalytic site-targeting PDE4B inhibitor (IC50 = 15 nM/PDE4B vs. 1.7 μM/PDE4D) that effectively prevents PDE4B-medicated cellular cAMP hydrolysis (150%/320% increased cAMP level with 100 nM/1 μM A-33 pre-treament in murine hippocampal HT-22 cells following 10 nM isoproterenol stimulation) in vitro and inhibits LPS-induced TNF-α production in mice in vivo (ID50 = 14 mg/kg p.o.). When administered via intraperitoneal injection, A-33 improves cognitive function in a rat model of traumatic brain injury (0.3 mg/kg i.p.) and exhibits antidepressant property in mice (0.3-1 mg/kg i.p.) in vivo.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
David J Titus et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 36(27), 7095-7108 (2016-07-08)
Learning and memory impairments are common in traumatic brain injury (TBI) survivors. However, there are no effective treatments to improve TBI-induced learning and memory impairments. TBI results in decreased cAMP signaling and reduced cAMP-response-element binding protein (CREB) activation, a critical
Timothy J Hagen et al.
Bioorganic & medicinal chemistry letters, 24(16), 4031-4034 (2014-07-08)
In this study we report a series of triazine derivatives that are potent inhibitors of PDE4B. We also provide a series of structure activity relationships that demonstrate the triazine core can be used to generate subtype selective inhibitors of PDE4B
Kenji Naganuma et al.
Bioorganic & medicinal chemistry letters, 19(12), 3174-3176 (2009-05-19)
In this study the first PDE4B selective inhibitor is described. Optimization of lead 2-arylpyrimidine derivatives afforded a series of potent PDE4B inhibitors with >100-fold selectivity over the PDE4D isozyme. With a good pharmacokinetic profile, a selected compound exhibited potent anti-inflammatory
David Fox et al.
Cellular signalling, 26(3), 657-663 (2013-12-24)
Phosphodiesterase-4B (PDE4B) regulates the pro-inflammatory Toll Receptor -Tumor Necrosis Factor α (TNFα) pathway in monocytes, macrophages and microglial cells. As such, it is an important, although under-exploited molecular target for anti-inflammatory drugs. This is due in part to the difficulty
Chong Zhang et al.
Scientific reports, 7, 40115-40115 (2017-01-06)
Inhibition of cyclic AMP (cAMP)-specific phosphodiesterase 4 (PDE4) has been proposed as a potential treatment for a series of neuropsychological conditions such as depression, anxiety and memory loss. However, the specific involvement of each of the PDE4 subtypes (PDE4A, 4B
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務