推薦產品
一般說明
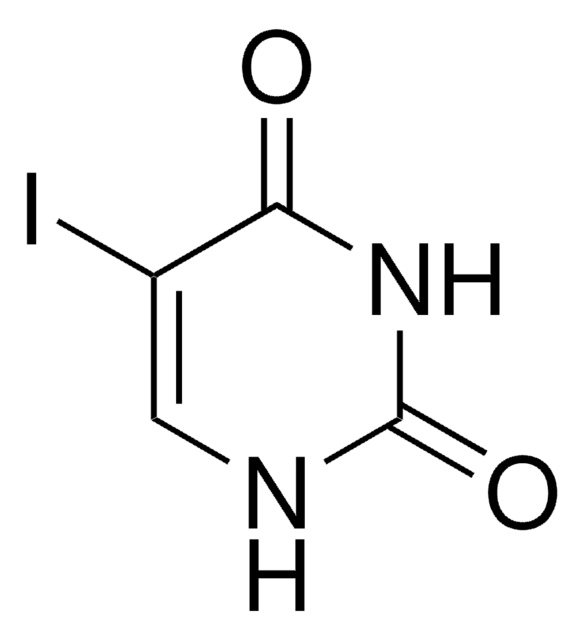
5-Iodocytosine is a modified pyrimidine used in the synthesis of molecules such as pyrrolocytosine and biologically active derivatives.[1]
應用
5-Iodocytosine has been used as iodinated nucleotide with the single crystals of the 3D DNA designed motif for single anomalous dispersion (SAD) studies.[2]
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
T S Heuer et al.
Biochemistry, 36(35), 10655-10665 (1997-09-02)
The virally encoded integrase protein carries out retroviral integration, and to do so, it must make specific interactions with both viral and target DNA sequences. The retroviral integrase has three domains: an amino-terminal region of about 50 amino acids that
Chemistry for the synthesis of nucleobase-modified peptide nucleic acid
Hudson RHE, et al.
Pure and Applied Chemistry. Chimie Pure Et Appliquee, 76(7-8), 1591-1598 (2004)
R H E Hudson et al.
Nucleosides, nucleotides & nucleic acids, 24(5-7), 581-584 (2005-10-27)
We have employed a tandem Sonogashira/annulation reaction between 5-iodocytosine derivatives and terminal alkynes to yield the fluorescent bicyclic nucleobase pyrrolcytosine. Pyrrolocytosine bearing substituents only on the pyrrole ring are conveniently synthesized from 5-iodocytosine. Water soluble pyrrolocytosines are being investigated as
Y Yoshimura et al.
Bioorganic & medicinal chemistry, 8(7), 1545-1558 (2000-09-08)
As part of our ongoing investigation of the synthesis of biologically interesting 2'-modified-4'-thionucleosides, we synthesized 2'-deoxy-2'-fluoro-4'-thioarabinofuranosylpyrimidine and -purine nucleosides, and evaluated their antiviral and antitumor activities. In the pyrimidine series, beta-anomers of 5-ethyluracil, 5-iodouracil, 5-chloroethyluracil, and 5-iodocytosine derivatives showed potent
S Harinipriya et al.
Journal of colloid and interface science, 250(1), 201-212 (2005-11-18)
The two-dimensional condensation behavior exhibited by nucleobases at a mercury/aqueous solution interface is analyzed on the basis of their hydrogen-bonded quadruplex structures, and the experimentally observed critical temperatures are rationalized incorporating different types of hydrogen bonding, surface coordination effects, and
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務