推薦產品
生物源
synthetic (organic)
化驗
≥93% (TLC)
形狀
powder
光學活性
[α]25/D 200 to 225 °, c = 0.5% (w/v) in chloroform
包含
1% CaCO3 as stabilizer
顏色
white to off-white
溶解度
chloroform: soluble 5 mg/mL
運輸包裝
dry ice
儲存溫度
−20°C
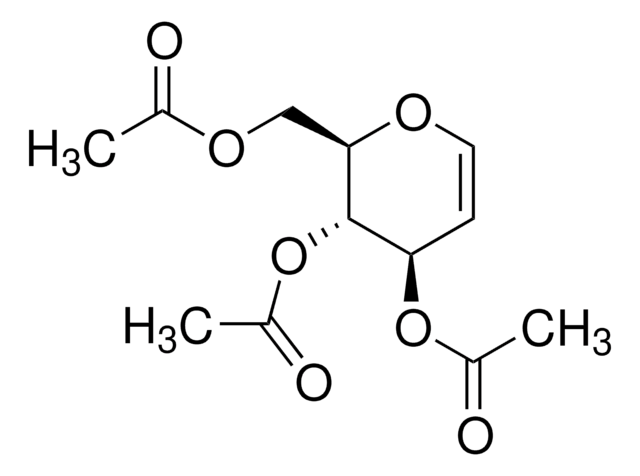
SMILES 字串
CC(=O)OC[C@H]1O[C@H](Br)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H]1OC(C)=O
InChI
1S/C14H19BrO9/c1-6(16)20-5-10-11(21-7(2)17)12(22-8(3)18)13(14(15)24-10)23-9(4)19/h10-14H,5H2,1-4H3/t10-,11+,12+,13-,14+/m1/s1
InChI 密鑰
CYAYKKUWALRRPA-HTOAHKCRSA-N
尋找類似的產品? 前往 產品比較指南
相關類別
應用
2,3,4,6-Tetra-O-acetyl-α-D-galactopyranosyl bromide is used as an organic condensation reagent for chemistries such as N- and S- galactosylation reactions and the synthesis of spacer-equipped phosphorylated di-, tri- and tetrasaccharide fragments of the O-specific polysaccharides.
其他說明
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Abdel Kader Mansour et al.
Nucleosides, nucleotides & nucleic acids, 22(9), 1825-1833 (2003-10-10)
The 1-(2,3,4,6-tetra-O-acetyl-beta-D-galactopyranosyl)-3-aryl-5-benzyl (or substituted benzyl)-1,2,4-triazin-6(1H)-/ones or thiones were prepared via galactosidation of 3-aryl-5-benzyl (or substituted benzyl)-1,2,4-triazin-6(1H)-/ones or thiones with 2,3,4,6-tetra-O-acetyl-alpha-D-galactopyranosyl bromide. The structure of the new galactosyl derivatives was based on both spectroscopic and chemical evidences.
Jiajia Qian et al.
The Journal of pharmacy and pharmacology, 71(6), 929-936 (2019-03-06)
Resveratrol (Res), a naturally occurring polyphenol, has shown pharmacological activities in treatment of liver diseases. However, the application of Res was limited by its poor bioavailability and liver targeting. Herein, 3-O-β-D-Galactosylated Resveratrol (Gal-Res) was synthesized by structural modification of Res
David J Claffey et al.
Carbohydrate research, 339(14), 2433-2440 (2004-09-25)
Condensation of 2,3,4,6-tetra-O-acetyl-alpha-D-glucopyranosyl-, 2,3,4-tri-O-acetyl-alpha-D-xylopyranosyl- and of 2,3,4,6-tetra-O-acetyl-alpha-D-galactopyranosyl bromides with l,4:3,6-dianhydro-D-glucitol under Koenigs-Knorr conditions, and using the Helferich modification of the reaction showed regioselectivity in glysosylation at C-5 of isosorbide.
Bart Ruttens et al.
Carbohydrate research, 341(9), 1077-1080 (2006-05-03)
The synthesis of oligosaccharide fragments of the O-specific polysaccharide of Vibrio cholerae O139 containing a 4,6-cyclic phosphate galactose residue linked to GlcNAc is described. 8-Azido-3,6-dioxaoctyl 2,3,4,6-tetra-O-acetyl-beta-D-galactopyranosyl-(1-->3)-2-acetamido-4,6-O-benzylidene-2-deoxy-beta-D-glucopyranoside, obtained by condensation of 2,3,4,6-tetra-O-acetyl-alpha-D-galactopyranosyl bromide and 8-azido-3,6-dioxaoctyl 2-acetamido-4,6-O-benzylidene-2-deoxy-beta-D-glucopyranoside, was converted to 8-azido-3,6-dioxaoctyl 3-O-benzyl-beta-D-galactopyranosyl-(1-->3)-2-acetamido-6-O-benzyl-2-deoxy-beta-D-glucopyranoside
Ahmed I Khodair et al.
Carbohydrate research, 346(18), 2831-2837 (2011-11-18)
N- and S-galactosylation was carried out via the reaction of 5-((Z)-arylidene)-2-thioxo-4-thiazolidinones with 2,3,4,6-tetra-O-acetyl-α-d-galactopyranosyl bromide under alkaline conditions or under silylation conditions. Deacetylation of the N-galactosylation products was performed with concentrated hydrochloric acid in methanol (3.5%) or sodium methoxide in methanol
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務