全部照片(1)
About This Item
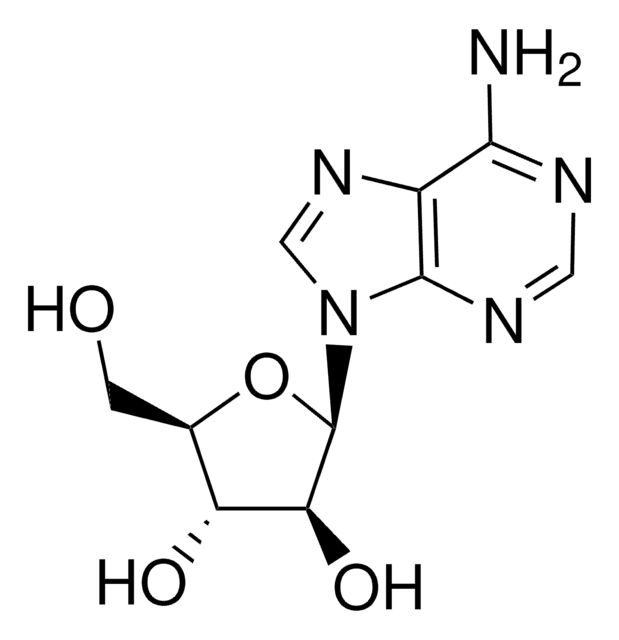
經驗公式(希爾表示法):
C10H13N5O5 · xH2O
CAS號碼:
分子量::
283.24 (anhydrous basis)
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.77
暫時無法取得訂價和供貨情況
推薦產品
化驗
≥98% (HPLC)
形狀
solid
溶解度
DMSO: >10 mg/mL
H2O: insoluble
儲存溫度
2-8°C
SMILES 字串
NC1=Nc2c(ncn2[C@@H]3O[C@H](CO)[C@@H](O)[C@@H]3O)C(=O)N1
InChI
1S/C10H13N5O5/c11-10-13-7-4(8(19)14-10)12-2-15(7)9-6(18)5(17)3(1-16)20-9/h2-3,5-6,9,16-18H,1H2,(H3,11,13,14,19)/t3-,5-,6+,9-/m1/s1
InChI 密鑰
NYHBQMYGNKIUIF-FJFJXFQQSA-N
應用
Ara-G is converted by cellular kinases to the active 5′-triphosphate, Ara-GTP. This active form of Ara-G induces apoptosis and inhibits DNA synthesis. Ara-G is also an antineoplastic and antimetabolite.
生化/生理作用
Ara-G is an inducer of apoptosis; inhibitor of DNA synthesis; antineoplastic; and antimetabolite.
Ara-G is an inducer of apoptosis; inhibitor of DNA synthesis; antineoplastic; and antimetabolite. Ara-G is converted by cellular kinases to the active 5′-triphosphate, Ara-GTP. Incorporation of Ara-GTP into DNA leads to inhibition of DNA synthesis and apoptosis.
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
從最近期的版本中選擇一個:
分析證明 (COA)
Lot/Batch Number
Sophie Curbo et al.
Reviews on recent clinical trials, 1(3), 185-192 (2008-05-14)
GW506U78 or nelarabine (Glaxo-SmithKline) is a nucleoside analog that is rapidly converted by cells of lymphoid lineage to its corresponding arabinosylguanine nucleotide triphosphate (araGTP). The triphosphate form of araG acts as a substrate for DNA polymerases and araG gets incorporated
C Zhu et al.
FEBS letters, 474(2-3), 129-132 (2000-06-06)
The anti-leukemic nucleoside analogs 1-beta-D-arabinofuranosylcytosine (araC) and 9-beta-D-arabinofuranosylguanine (araG) are dependent on intracellular phosphorylation for pharmacological activity. AraC is efficiently phosphorylated by deoxycytidine kinase (dCK). Although araG is phosphorylated by dCK in vitro, it is a preferred substrate of mitochondrial
Carlos O Rodriguez et al.
Cancer research, 62(11), 3100-3105 (2002-05-31)
The prodrug of 9-beta-D-arabinosylguanine (ara-G), nelarabine, demonstrated efficacy against T-cell acute lymphoblastic leukemia, and its effectiveness correlated with the accumulation of the triphosphate form (ara-GTP). Although in vitro investigations using purified deoxycytidine kinase (dCK) or deoxyguanosine kinase (dGK) suggested that
Luigi Leanza et al.
Experimental cell research, 316(20), 3443-3453 (2010-07-07)
The deoxyguanosine (GdR) analog guanine-ß-d-arabinofuranoside (araG) has a specific toxicity for T lymphocytes. Also GdR is toxic for T lymphocytes, provided its degradation by purine nucleoside phosphorylase (PNP) is prevented, by genetic loss of PNP or by enzyme inhibitors. The
Stacey L Berg et al.
Cancer chemotherapy and pharmacology, 59(6), 743-747 (2006-09-06)
Nelarabine is a water-soluble prodrug of the cytotoxic deoxyguanosine analog ara-G, to which it is rapidly converted in vivo by adenosine deaminase. Nelarabine has shown activity in the treatment of T-cell malignancies, especially T-cell acute lymphoblastic leukemia. Preliminary data suggested
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務