推薦產品
化驗
≥95.0% (HPLC)
形狀
powder or crystals
應用
metabolomics
vitamins, nutraceuticals, and natural products
SMILES 字串
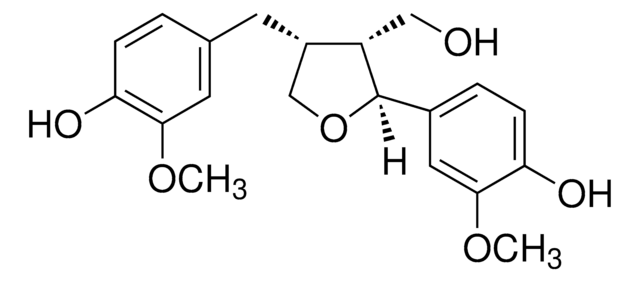
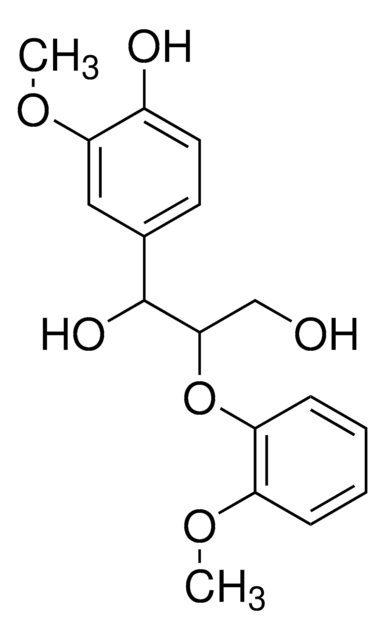
COc1cc(ccc1O)[C@H]2OC[C@H]3[C@@H]2CO[C@@H]3c4ccc(O)c(OC)c4
InChI
1S/C20H22O6/c1-23-17-7-11(3-5-15(17)21)19-13-9-26-20(14(13)10-25-19)12-4-6-16(22)18(8-12)24-2/h3-8,13-14,19-22H,9-10H2,1-2H3/t13-,14-,19+,20+/m0/s1
InChI 密鑰
HGXBRUKMWQGOIE-AFHBHXEDSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
應用
Pinoresinol has been used:
- as a reference standard for qualitative and quantitative analysis of lignans from Triticale (X Triticosecale Wittmack) grains using ultra-performance liquid chromatography (UPLC) with photodiode and mass TQD detectors[2]
- as an enterolignan precursor to study its estrogenic activity on the proliferation of human breast cancer MCF-7 cells[3]
- as a reference standard for lignan analysis of Sesamum indicum L. seeds[4]
生化/生理作用
松脂素存在于包括药用植物在内的多种植物中,如水生接骨木、杜仲、安息香属、连翘以及特级初榨橄榄油中。其中的接骨木属广泛分布于欧洲、亚洲和北非,并已在传统医学中作为止痛药、抗病毒药、抗炎药、止血药和利尿药用于瘀伤、骨折和水肿。松脂素可通过引起真菌质膜的损伤而显示出有效的抗真菌特性。它通过抑制TNF-α的产生(很可能是通过抑制NF- κB和AP-1)而发挥出抗氧化和抗炎的作用。
通过抑制TNF-α的产生而发挥出抗氧化和抗炎的作用。
包裝
Bottomless glass bottle. Contents are inside inserted fused cone.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Frank C Schroeder et al.
Proceedings of the National Academy of Sciences of the United States of America, 103(42), 15497-15501 (2006-10-13)
Pinoresinol, a lignan of wide distribution in plants, is found to occur as a minor component in the defensive secretion produced by glandular hairs of caterpillars of the cabbage butterfly, Pieris rapae. The compound or a derivative is appropriated by
Kishan Chandra et al.
Biotechnology reports (Amsterdam, Netherlands), 22, e00336-e00336 (2019-04-25)
Members of Cytochromes P450 super family of enzymes catalyse important biochemical reactions in plants. Some of these reactions are so important that they contribute to enormous chemical diversity seen in plants. Many unique secondary metabolites formed by mediation of these
Lignans in triticale grain and triticale products
Makowska A, et al.,
Journal of Cereal Science, 93, 102939-102939 (2020)
Yun Zhu et al.
PloS one, 12(2), e0171390-e0171390 (2017-02-06)
Mammalian lignans or enterolignans are metabolites of plant lignans, an important category of phytochemicals. Although they are known to be associated with estrogenic activity, cell signaling pathways leading to specific cell functions, and especially the differences among lignans, have not
Hyo Won Jung et al.
Neuroscience letters, 480(3), 215-220 (2010-07-06)
The activation of microglia plays an important role in a variety of brain disorders by the excessive production of inflammatory mediators such as nitric oxide (NO), prostaglandin E(2) (PGE(2)) and proinflammatory cytokines. We investigated here whether pinoresinol isolated from the
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務