全部照片(1)
About This Item
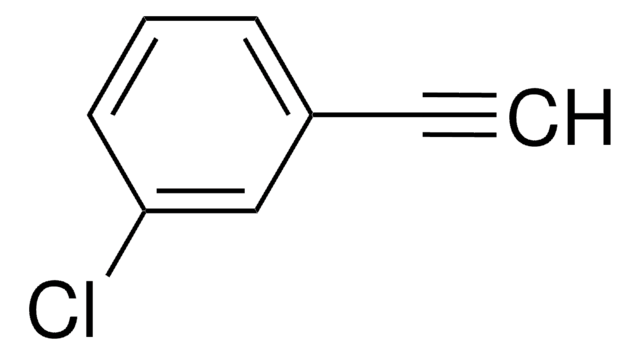
經驗公式(希爾表示法):
C12H14O2
CAS號碼:
分子量::
190.24
EC號碼:
MDL號碼:
分類程式碼代碼:
12352204
PubChem物質ID:
NACRES:
NA.25
暫時無法取得訂價和供貨情況
推薦產品
化驗
99%
形狀
liquid
折射率
n20/D 1.56 (lit.)
bp
68 °C/0.1 mmHg (lit.)
密度
1.052 g/mL at 25 °C (lit.)
應用
agriculture
environmental
SMILES 字串
COc1ccc2C=CC(C)(C)Oc2c1
InChI
1S/C12H14O2/c1-12(2)7-6-9-4-5-10(13-3)8-11(9)14-12/h4-8H,1-3H3
InChI 密鑰
CPTJXGLQLVPIGP-UHFFFAOYSA-N
Atsushi Yaguchi et al.
Journal of agricultural and food chemistry, 57(3), 846-851 (2009-02-05)
Inhibitors of deoxynivalenol production by Fusarium graminearum are useful for protecting crops from deoxynivalenol contamination. We isolated precocenes and piperitone from the essential oils of Matricaria recutita and Eucalyptus dives, respectively, as specific inhibitors of the production of 3-acetyldeoxynivalenol, a
V Ravindranath et al.
Biochemical pharmacology, 36(4), 441-446 (1987-02-15)
The mechanism of the hepatotoxicity of precocene I has been investigated in male, Sprague-Dawley rats. Administration of a single dose of precocene I caused a large depletion of liver glutathione (GSH) levels that was both time and dose dependent. Concomitant
A Fodor et al.
General and comparative endocrinology, 74(1), 18-31 (1989-04-01)
Precocenes (PI and PII) and 114 of their analogs (PAs) were synthetized and tested on C. remanei embryos for their precocene-like (P-like) activities resulting in unusual development at sublethal doses. The P-like activity was quantitated by plotting the probit of
Angela M Bernard et al.
Organic letters, 4(15), 2565-2567 (2002-07-19)
[reaction: see text] The thionium ion, generated through a cyclopropylcarbinyl-cyclobutyl ring expansion, is, for the first time, intramolecularly intercepted by activated aromatic rings to generate new versatile 2a-methyl-8b-(phenylsulfanyl-1,2a,3,8b-tetrahydro-2H-cyclobuta[c]chromenes.
M I Baldellou et al.
Revista espanola de fisiologia, 42(3), 315-317 (1986-09-01)
Antigonadotropic activities of Precocene 1 (P1), Precocene 2 (P2) and Ethoxyprecocene 2 (EP2) on the seed bug Oxycarenus lavaterae (F.) (Heteroptera, Lygaeidae), are reported. EP2 proved to be the most active compound followed by P2 and P1, which agrees with
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務