推薦產品
等級
pharmaceutical primary standard
API 家族
rifaximin
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
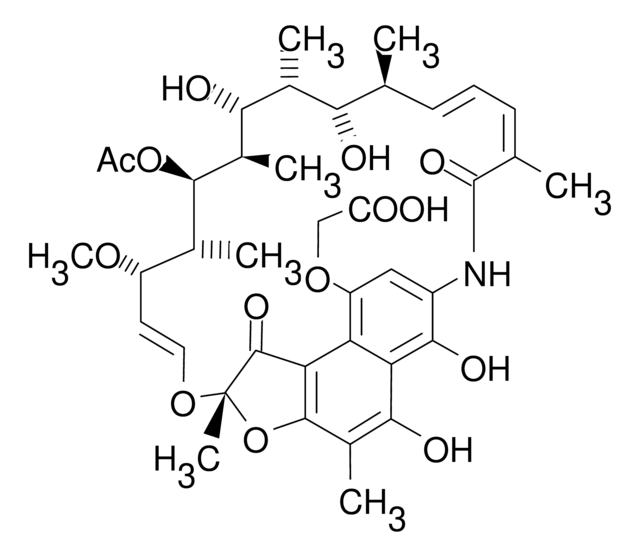
CO[C@H]1\C=C\O[C@@]2(C)Oc3c(C)c(O)c4c(O)c(NC(=O)C(C)=C\C=C\[C@@H](C)[C@@H](O)[C@@H](C)[C@H](O)[C@H](C)[C@H]([C@H]1C)C(=O)OC)c5c(nc6cc(C)ccn56)c4c3C2=O
InChI
1S/C43H51N3O11/c1-19-14-16-46-27(18-19)44-32-29-30-37(49)25(7)39-31(29)40(51)43(8,57-39)56-17-15-26(54-9)22(4)28(42(53)55-10)23(5)36(48)24(6)35(47)20(2)12-11-13-21(3)41(52)45-33(34(32)46)38(30)50/h11-18,20,22-24,26,28,35-36,47-50H,1-10H3,(H,45,52)/b12-11+,17-15+,21-13-/t20-,22+,23-,24-,26+,28+,35-,36-,43+/m1/s1
InChI 密鑰
HIYLTQREEOINNF-HTEWPBCCSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Rifaximin EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
S S Belous et al.
Eksperimental'naia i klinicheskaia gastroenterologiia = Experimental & clinical gastroenterology, (3)(3), 63-68 (2013-12-04)
One of the important problems in the treatment of patients with inflammatory bowel disease are the persistent complaints of pain, abdominal distention, frequent stools, excretion of mucus with faeces with the presence of endoscopic or roentgenologic remission in the damaged
David J Farrell
Journal of clinical gastroenterology, 47(3), 205-211 (2013-01-24)
Irritable bowel syndrome (IBS), a chronic, nonfatal illness is commonly encountered in clinical practice; however, treatment options are limited and often ineffectual. Despite this, there is increasing evidence that bacterial overgrowth in the bowel (dysbiosis) may be an etiological factor
Anastasia Rivkin et al.
Clinical therapeutics, 33(7), 812-827 (2011-07-12)
Rifaximin is a nonabsorbable oral antibiotic that acts locally in the gastrointestinal tract with minimal systemic adverse effects. Rifaximin received new labeling for reduction in the risk of the recurrence of overt hepatic encephalopathy (HE) in patients with advanced liver
Rima I El-Herte et al.
Scandinavian journal of infectious diseases, 44(3), 228-230 (2011-11-15)
Clostridium difficile colitis infection is on the rise and is considerably increasing the duration of hospital stay, as well as healthcare costs. The management of C. difficile colitis has become more challenging with the increasing failure of therapeutic response to
Kenneth R Lawrence et al.
Pharmacotherapy, 28(8), 1019-1032 (2008-07-29)
To review the effectiveness and safety of rifaximin for the treatment of hepatic encephalopathy. A literature search was conducted of MEDLINE (1966-September 2007), the Cochrane Database of Systematic Reviews (1995-2007), and the Cochrane Hepato-Biliary Group Reviews (2003-2007). English-language articles identified
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務