全部照片(1)
About This Item
經驗公式(希爾表示法):
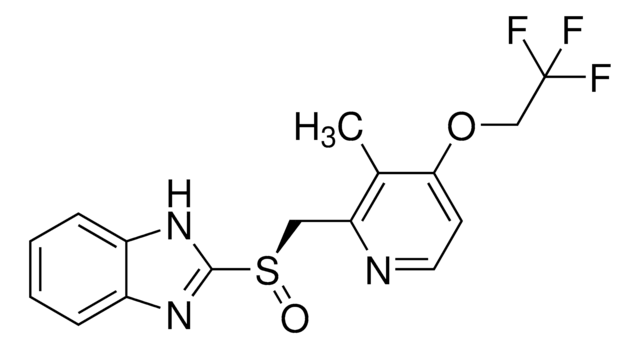
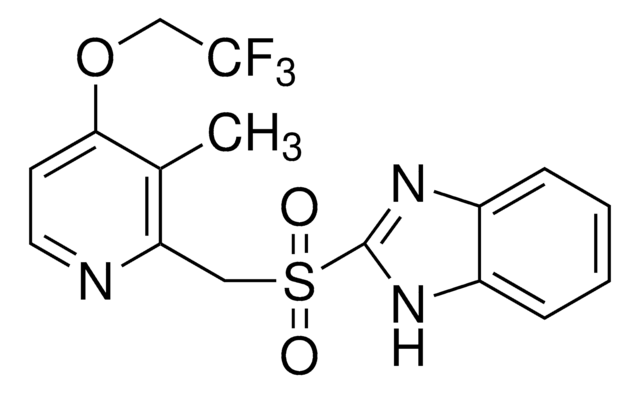
C16H14F3N3O2S
CAS號碼:
分子量::
369.36
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推薦產品
等級
pharmaceutical primary standard
API 家族
lansoprazole
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
Cc1c(CS(=O)c2nc3ccccc3[nH]2)nccc1OCC(F)(F)F
InChI
1S/C16H14F3N3O2S/c1-10-13(20-7-6-14(10)24-9-16(17,18)19)8-25(23)15-21-11-4-2-3-5-12(11)22-15/h2-7H,8-9H2,1H3,(H,21,22)
InChI 密鑰
MJIHNNLFOKEZEW-UHFFFAOYSA-N
基因資訊
human ... ATP4A(495) , ATP4B(496)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Lansoprazole EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Hiroshi Satoh
Current pharmaceutical design, 19(1), 67-75 (2012-09-07)
The proton pump inhibitors (PPIs) lansoprazole (LPZ) and omeprazole (OPZ) have been widely used for more than 20 years in the treatment of acid-related diseases such as gastro-duodenal ulcers and reflux esophagitis. Both LPZ and OPZ are derivatives of 2-[(2-
Yun Jeong Lim et al.
Digestion, 86(2), 171-177 (2012-08-22)
Proton pump inhibitors (PPIs) are widely used to prevent nonsteroidal anti-inflammatory drug (NSAID)-induced peptic ulcers. NSAIDs produce small intestinal injury and some PPIs have been reported to protect against NSAID-induced small bowel injury in rats. The aim of this study
Grace Chai et al.
Pediatrics, 130(1), 23-31 (2012-06-20)
To describe trends in outpatient prescription drug utilization in US children and the changes in major areas of pediatric therapeutic use for the years 2002 through 2010. Large prescription databases (the IMS Vector One: National and Total Patient Tracker) were
Jyh-Ming Liou et al.
Lancet (London, England), 381(9862), 205-213 (2012-11-20)
Whether sequential treatment can replace triple therapy as the standard treatment for Helicobacter pylori infection is unknown. We compared the efficacy of sequential treatment for 10 days and 14 days with triple therapy for 14 days in first-line treatment. For
D A Peura et al.
Alimentary pharmacology & therapeutics, 37(8), 810-818 (2013-03-05)
Higher body mass index (BMI) is a recognised risk factor for gastro-oesophageal reflux disease (GERD). Data regarding the impact of BMI on proton pump inhibitor (PPI) therapy are conflicting. To assess the impact of BMI on baseline heartburn symptom severity
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務