推薦產品
等級
pharmaceutical primary standard
API 家族
amlodipine
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
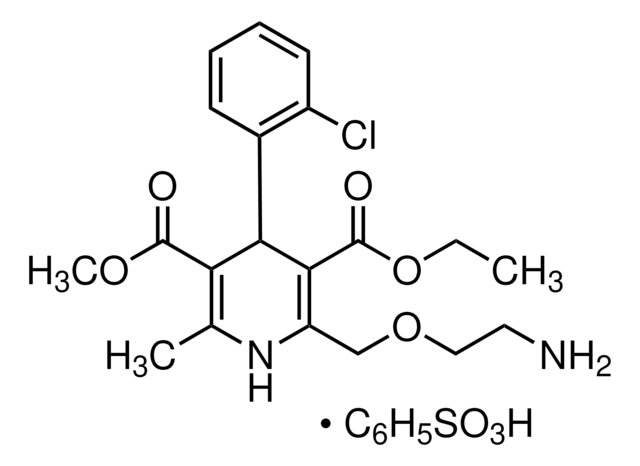
OS(=O)(=O)c1ccccc1.CCOC(=O)C2=C(COCCN)NC(C)=C(C2c3ccccc3Cl)C(=O)OC
InChI
1S/C20H25ClN2O5.C6H6O3S/c1-4-28-20(25)18-15(11-27-10-9-22)23-12(2)16(19(24)26-3)17(18)13-7-5-6-8-14(13)21;7-10(8,9)6-4-2-1-3-5-6/h5-8,17,23H,4,9-11,22H2,1-3H3;1-5H,(H,7,8,9)
InChI 密鑰
ZPBWCRDSRKPIDG-UHFFFAOYSA-N
基因資訊
human ... CACNA1C(775) , CACNA1D(776) , CACNA1F(778) , CACNA1S(779)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Amlodipine besylate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
氨氯地平是一种 L 型钙通道阻滞剂。氨氯地平属于一类心血管药物,作用于 Ca V 1 或 L 型的电压门控钙通道。氨氯地平还具有降压和抗心绞痛作用。其活性主要存在于 (-)-异构体中。氨氯地平抑制人表皮样癌细胞 A431 的生长,对雄性大鼠有抗生殖作用。
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
產品號碼
描述
訂價
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
客戶也查看了
S Neldam et al.
International journal of clinical practice, 67(9), 843-852 (2013-08-21)
Rapid and sustained blood pressure (BP) goal attainment is important to reduce cardiovascular risk. Initial use of combination therapy may improve BP goal attainment. The Boehringer Ingelheim trial database was searched for randomised, double-blind studies comparing telmisartan/amlodipine combination therapy with
Juliano Lara Fernandes et al.
The American journal of medicine, 126(9), 834-837 (2013-07-09)
Iron chelation therapy in patients with thalassemia major may not prevent iron overload in all organs, especially those in which iron enters cells through specific calcium channels. We designed a controlled pilot study to assess the potential of the calcium
Giuseppe Derosa et al.
Journal of the American Society of Hypertension : JASH, 7(1), 32-39 (2013-01-17)
The purpose of this study was to evaluate a fixed olmesartan/amlodipine combination on blood pressure control, lipid profile, insulin sensitivity, and some inflammatory markers compared with single-drug monotherapy. A total of 276 hypertensive patients were randomly assigned to olmesartan 20
Mehmet Gumustas et al.
Journal of AOAC International, 96(4), 751-757 (2013-09-05)
A stability-indicating RP-LC assay method was developed for the simultaneous determination of the cardiovascular drugs amlodipine and perindopril in the presence of degradation products generated from forced decomposition studies. The developed method is applicable for the determination of related substances
László Bajnok
Orvosi hetilap, 154(7), 243-247 (2013-02-12)
From the evaluated ONTARGET, ALTITUDE, ACCOMPLISH, ROADMAP, and ACCORD-BP studies a conclusion can be drawn that though microalbuminuria/proteinuria is a strong epidemiological biomarker, in interventional studies it is not necessarily a reliable surrogate endpoint as actual renal function may change
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務