推薦產品
等級
analytical standard, for drug analysis
品質等級
化驗
≥95% (TLC)
形狀
solid
分子量
apparent mol wt 835.1
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
forensics and toxicology
pharmaceutical (small molecule)
veterinary
格式
neat
SMILES 字串
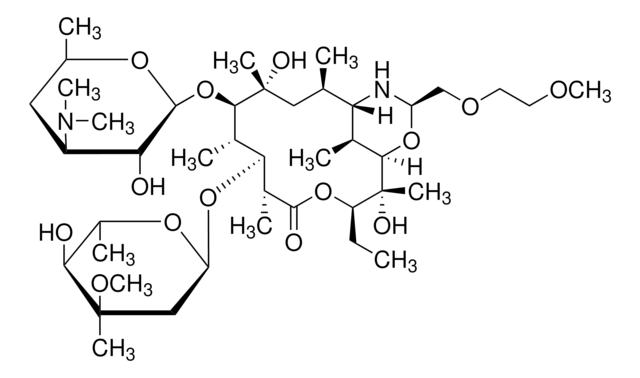
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C4N[C@@H](COCCOC)OC([C@H]4C)[C@]1(C)O
InChI
1S/C42H78N2O14/c1-15-29-42(10,49)37-24(4)32(43-30(56-37)21-52-17-16-50-13)22(2)19-40(8,48)36(58-39-33(45)28(44(11)12)18-23(3)53-39)25(5)34(26(6)38(47)55-29)57-31-20-41(9,51-14)35(46)27(7)54-31/h22-37,39,43,45-46,48-49H,15-21H2,1-14H3/t22-,23-,24+,25+,26-,27+,28+,29-,30-,31+,32+,33-,34+,35+,36-,37-,39+,40-,41+,42-/m1/s1
InChI 密鑰
WLOHNSSYAXHWNR-KZYCBHIHSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
應用
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務