推薦產品
等級
pharmaceutical primary standard
API 家族
busulfan
製造商/商標名
EDQM
mp
114-117 °C (lit.)
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
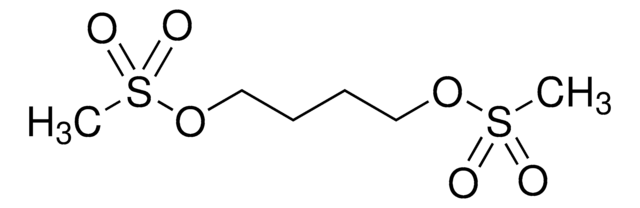
CS(=O)(=O)OCCCCOS(C)(=O)=O
InChI
1S/C6H14O6S2/c1-13(7,8)11-5-3-4-6-12-14(2,9)10/h3-6H2,1-2H3
InChI 密鑰
COVZYZSDYWQREU-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Carc. 1A - Muta. 1B - Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
客戶也查看了
Edward A Copelan et al.
Blood, 122(24), 3863-3870 (2013-09-26)
Cyclophosphamide combined with total body irradiation (Cy/TBI) or busulfan (BuCy) are the most widely used myeloablative conditioning regimens for allotransplants. Recent data regarding their comparative effectiveness are lacking. We analyzed data from the Center for International Blood and Marrow Transplant
Je-Hwan Lee et al.
Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 31(6), 701-709 (2012-11-07)
We conducted a phase III randomized clinical trial to compare two myeloablative conditioning regimens for allogeneic hematopoietic cell transplantation (HCT) in patients with leukemia and myelodysplastic syndrome. After randomization, 64 patients received busulfan (3.2 mg/kg per day × 4 days)
Christopher Bredeson et al.
Blood, 122(24), 3871-3878 (2013-10-02)
We conducted a prospective cohort study testing the noninferiority of survival of ablative intravenous busulfan (IV-BU) vs ablative total body irradiation (TBI)-based regimens in myeloid malignancies. A total of 1483 patients undergoing transplantation for myeloid malignancies (IV-BU, N = 1025;
Arnon Nagler et al.
Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 31(28), 3549-3556 (2013-08-28)
Cyclophosphamide (Cy) combined with total-body irradiation (TBI) or with busulfan (Bu) are currently the most common myeloablative regimens used in allogeneic stem-cell transplantation (alloSCT) in adults with acute myelogenous leukemia (AML). Intravenous (IV) Bu has more predictable bioavailability and a
J S McCune et al.
Clinical pharmacokinetics, 39(2), 155-165 (2000-09-08)
High dosage busulfan (1 mg/kg orally every 6 hours x 16 doses) is frequently used in preparative regimens for haemopoietic stem cell transplantation (HSCT). Busulfan is well absorbed after oral administration, exhibits low protein binding and is metabolised through conjugation
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務