推薦產品
等級
derivatization grade ((chiral))
for chiral derivatization
品質等級
化驗
≥99.0% (sum of enantiomers, TLC)
≥99.0%
形狀
powder
光學活性
[α]20/D +56±2°, c = 1% in acetone
光學純度
enantiomeric ratio: ≥99.5:0.5 (HPLC)
品質
LiChropur™
技術
HPLC: suitable
儲存溫度
2-8°C
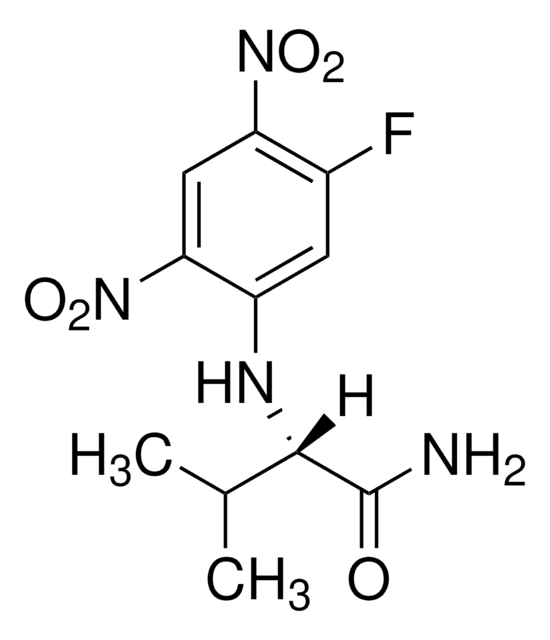
SMILES 字串
C[C@H](Nc1cc(F)c(cc1[N+]([O-])=O)[N+]([O-])=O)C(N)=O
InChI
1S/C9H9FN4O5/c1-4(9(11)15)12-6-2-5(10)7(13(16)17)3-8(6)14(18)19/h2-4,12H,1H3,(H2,11,15)/t4-/m0/s1
InChI 密鑰
NEPLBHLFDJOJGP-BYPYZUCNSA-N
尋找類似的產品? 前往 產品比較指南
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
Chirality determination of unusual amino acids using precolumn derivatization and liquid chromatography-electrospray ionization mass spectrometry.
Hess S
Journal of Chromatography A, 1035(2), 211-219 (2004)
Evan W Rogers et al.
Journal of natural products, 68(6), 891-896 (2005-06-25)
The absolute configurations of fistularin-3, 11-epi-fistularin-3, and a related bis-oxazolidinone were determined by microscale hydrolysis followed by derivatization with 1-fluoro-2,4-dinitrophenyl-5-l-alaninamide. Samples of fistularin-3 from Verongid marine sponges collected in the Great Barrier Reef (Australia), Baía de Todos os Santos (Brazil)
D R Goodlett et al.
Journal of chromatography. A, 707(2), 233-244 (1995-07-21)
A high-performance liquid chromatography-electrospray ionization-mass spectrometric (LC-ESI-MS) method is presented that allows rapid and accurate determination of amino acid chiral purity in a peptide. Peptides are hydrolyzed in hydrochloric acid-d1/acetic acid-d4 and then converted to diastereomers by derivatization with 1-fluoro-2,4-dinitrophenyl-5-L-alanine
E Harth-Fritschy et al.
The journal of peptide research : official journal of the American Peptide Society, 50(6), 415-420 (1998-01-24)
Esterification of glycosylated serine and cysteine derivatives with a 4-alkoxybenzyl alcohol (Wang) resin is described. The classical methods of ester bond formation (symmetrical anhydride, 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate [TBTU]/4-dimethylaminopyridine [DMAP] with or without 1-hydroxybenzotriazole [HOBT], pentafluorophenyl [Pfp] esters gave high percentages of
D B Goodnough et al.
Journal of chromatography. B, Biomedical applications, 672(2), 290-294 (1995-10-20)
D-Serine has recently been described to be present in the brain at high concentrations. However, while prior research has demonstrated that L-phosphoserine is the major precursor of L-serine in the brain, the possible role of D-phosphoserine as the direct precursor
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務