5.30487
StemSelect PD 0332991
同義詞:
StemSelect PD 0332991, 6-Acetyl-8-cyclopentyl-5-methyl-2-(5-(piperazin-1-yl)pyridin-2-ylamino)pyrido[2,3-d]pyrimidin-7(8H)-one, HCl, Cdk4/Cdk6 Inhibitor V, PD-0332991, HCl, PF-332991, HCl
登入查看組織和合約定價
全部照片(1)
About This Item
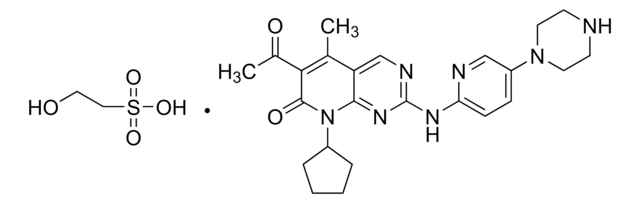
經驗公式(希爾表示法):
C24H29N7O2 · xHCl
CAS號碼:
分子量::
447.53 (free base basis)
分類程式碼代碼:
12352200
NACRES:
NA.77
推薦產品
化驗
≥97% (HPLC)
品質等級
形狀
liquid
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
avoid repeated freeze/thaw cycles
protect from light
顏色
yellow
溶解度
water: 10 mg/mL
儲存溫度
−70°C
InChI
1S/C24H29N7O2.ClH/c1-15-19-14-27-24(28-20-8-7-18(13-26-20)30-11-9-25-10-12-30)29-22(19)31(17-5-3-4-6-17)23(33)21(15)16(2)32;/h7-8,13-14,17,25H,3-6,9-12H2,1-2H3,(H,26,27,28,29);1H
InChI 密鑰
STEQOHNDWONVIF-UHFFFAOYSA-N
一般說明
A cell-permeable, orally available and brain permeant, non-toxic pyridopyrimidinone compound that acts as a potent, selective, reversible, ATP competitive inhibitor of Cdk4 and Cdk6 (IC50 = 11, 9, and 15 nM for Cdk4/D1, Cdk4/D3 and Cdk6/D2, respectively). Hence, it reduces retinoblastoma protein phosphorylation at Ser780/Ser795 (IC50 = 66 nM in MDA-435 cells) and arrests cell cycle at G1 phase. Acts as a cytostatic agent, but does induce apoptotic cell death when used alone. However, it potentiates the cytotoxicity of dexamethasone (>Cat. No. 265005), bortezomib (>Cat. No. 504314), and tamoxifen (Cat. No. 579000) in estrogen receptor (ER)-positive cell lines. Exhibits only a trivial inhibitory activity towards Cdk2/E2, Cdk2/A, Cdk1/B and Cdk5/p25 in a 36-kinase panel (IC50 >10 µM). Improves endoderm differentiation of late G1-human embryonic stem cells expressing Smad2 or Smad3 (~ 750 nM) and further enhances endoderm differentiation into hepatic and pancreatic progenitor cells. Shown to regress the growth of human breast tumor xenografts in murine models (~150 mg/kg, p.o., daily).
A cell-permeable, orally available and brain permeant, non-toxic pyridopyrimidinone compound that acts as a potent, selective, reversible, ATP competitive inhibitor of Cdk4 and Cdk6 (IC50 = 11, 9, and 15 nM for Cdk4/D1, Cdk4/D3 and Cdk6/D2, respectively). Hence, it reduces retinoblastoma protein phosphorylation at Ser780/Ser795 (IC50 = 66 nM in MDA-435 cells) and arrests cell cycle at G1 phase. Acts as a cytostatic agent, but does induce apoptotic cell death when used alone. However, it potentiates the cytotoxicity of dexamethasone (>Cat. No. 265005), bortezomib (>Cat. No. 504314), and tamoxifen (Cat. No. 579000) in estrogen receptor (ER)-positive cell lines. Exhibits only a trivial inhibitory activity towards Cdk2/E2, Cdk2/A, Cdk1/B and Cdk5/p25 in a 36-kinase panel (IC50 >10 µM). Improves endoderm differentiation of late G1-human embryonic stem cells expressing Smad2 or Smad3 (~ 750 nM) and further enhances endoderm differentiation into hepatic and pancreatic progenitor cells. Shown to regress the growth of human breast tumor xenografts in murine models (~150 mg/kg, p.o., daily).
Please note that the molecular weight for this compound is batch-specific due to variable water content.
Please note that the molecular weight for this compound is batch-specific due to variable water content.
生化/生理作用
Cell permeable: yes
Primary Target
Cdk4 & Cdk6
Cdk4 & Cdk6
Reversible: yes
包裝
Packaged under inert gas
警告
Toxicity: Standard Handling (A)
重構
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 6 months at -20°C.
其他說明
Pauklin, S. and Vallier, L., 2013. Cell155, 135.
Finn, R.S., et al. 2009. Breast Cancer Res.11, R17.
Menu, E., et al. 2008. Cancer Res.68, 5519.
Baughn, L.B., et al. 2006. Cancer Res.66, 7661.
Fry, D.W., et al. 2004. Mol. Cancer Ther.3, 1427.
Finn, R.S., et al. 2009. Breast Cancer Res.11, R17.
Menu, E., et al. 2008. Cancer Res.68, 5519.
Baughn, L.B., et al. 2006. Cancer Res.66, 7661.
Fry, D.W., et al. 2004. Mol. Cancer Ther.3, 1427.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務