推薦產品
等級
certified reference material
品質等級
形狀
liquid
包裝
ampule of 1 mL
製造商/商標名
Cerilliant®
drug control
Narcotic Licence Schedule B (Switzerland); psicótropo (Spain); Decreto Lei 15/93: Tabela IV (Portugal)
濃度
1 mg/mL in methanol
技術
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
應用
clinical testing
形式
single component solution
儲存溫度
−20°C
SMILES 字串
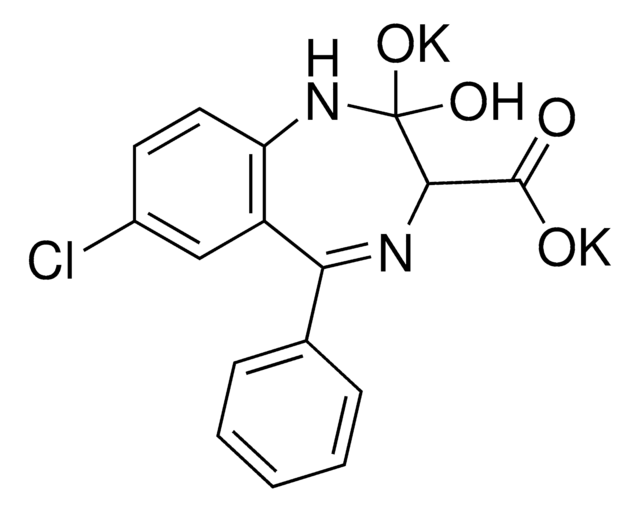
Clc1ccc2NC(=O)CN=C(c3ccccc3)c2c1
InChI
1S/C15H11ClN2O/c16-11-6-7-13-12(8-11)15(17-9-14(19)18-13)10-4-2-1-3-5-10/h1-8H,9H2,(H,18,19)
InChI 密鑰
AKPLHCDWDRPJGD-UHFFFAOYSA-N
一般說明
去甲西泮是一种苯二氮卓类药物,用于治疗焦虑症和恐慌症。该分析标准物质适用于LC/MS或GC/MS应用,以进行临床毒理学研究、法医分析或尿液药物检测。去甲西泮也是苯二氮卓类药物地西泮和氯氮卓的主要尿液代谢产物。
推薦產品
在我们的NMR在线平台ChemisTwin®上可以找到本品对应的数字化标准物质。您可使用ChemisTwin®上的数字等效品鉴定您的样品并进行定量分析(使用数字化外标)。可查看该物质的NMR谱图,只需点击几次鼠标,就能进行在线样品比对。欢迎点击了解更多,开启免费试用之旅。
法律資訊
CERILLIANT is a registered trademark of Merck KGaA, Darmstadt, Germany
相關產品
產品號碼
描述
訂價
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
標靶器官
Eyes,Central nervous system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
49.5 °F - closed cup
閃點(°C)
9.7 °C - closed cup
客戶也查看了
Joris C Verster et al.
Sleep medicine reviews, 17(2), 153-159 (2012-08-14)
The use of benzodiazepine receptor agonists can significantly impair driving performance. The aim of this review was to determine if there is a relation between blood concentrations of these drugs and the degree of driving impairment. A literature search was
Craig Lehmann et al.
Journal of clinical pharmacology, 48(4), 436-444 (2008-02-02)
A diazepam 10-mg autoinjector was evaluated in bioequivalence and dose proportionality studies; both involved 24 young, healthy subjects and used randomized, open-label, 2-treatment, 2-period crossover designs with a 3-week washout period between treatments. The bioequivalence study compared a single diazepam
Salvatore Millefiori et al.
Journal of molecular modeling, 17(2), 281-287 (2010-05-07)
Vertical ionization energies (VIEs) of medazepam, nordazepam and their molecular subunits have been calculated using the electron propagator method in the P3/CEP-31G* approximation. Vertical electron affinities (VEAs) have been obtained with a ∆SCF procedure at the DFT-B3LYP/6-31+G* level of theory.
Michael J Lamson et al.
Clinical drug investigation, 31(8), 585-597 (2011-07-05)
Acute repetitive seizures (ARS) are a debilitating part of episodic seizure activity that can sometimes progress to status epilepticus. Currently approved treatment that can be administered by non-medical personnel to patients with ARS is a diazepam rectal gel. While effective
Laura Mercolini et al.
Talanta, 80(1), 279-285 (2009-09-29)
Diazepam is frequently used as an adjuvant during antidepressant therapy. Recently, some studies have suggested that the treatment with benzodiazepines could have different efficacy in depressed patients as opposed to non-depressed ones. To clarify the matter, a study is currently
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務