推薦產品
描述
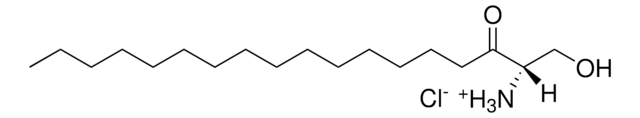
cholest-5-ene-3β,22(S)-diol-d7
化驗
>99% (TLC)
形狀
powder
包裝
pkg of 1 × 1 mg (700051P-1mg)
製造商/商標名
Avanti Research™ - A Croda Brand
運輸包裝
dry ice
儲存溫度
−20°C
一般說明
22(S)-hydroxycholesterol is an enantiomer of 22(R)-hydroxycholesterol.[1] 22(S)-hydroxycholesterol-d7 is a deuterated form of 22(S)-hydroxycholesterol.
應用
22(S)-hydroxycholesterol-d7 may be used as an internal standard in liquid chromatography with tandem mass spectrometry (LC-MS-MS) analysis of plasma low-density lipoprotein (LDL).[2]
生化/生理作用
22(S)-hydroxycholesterol (22(S)-HC) promotes glucose catabolism and uptake and is regarded as a potential target to treat type 2 diabetes.[3] 22(S)-HC also prevents the accumulation of lipids and lipid synthesis in hepatocytes and myotubes.[3] Unlike 22(R)-hydroxycholesterol, 22(S)-HC is not estrogenic and is not a ligand for liver X receptor (LXR).[1]
包裝
5 mL Amber Glass Screw Cap Vial (700051P-1mg)
法律資訊
Avanti Research is a trademark of Avanti Polar Lipids, LLC
儲存類別代碼
11 - Combustible Solids
Hiroyoshi Sato et al.
Bioscience, biotechnology, and biochemistry, 68(8), 1790-1793 (2004-08-24)
In order to test the estrogenic activity of sterol oxidation products from cholesterol and phytosterols, an estrogen-dependent gene expression assay was performed in estrogen receptor alpha-stably transformed HeLa cells. The ranking of the estrogenic potency of these compounds was different:
Myung-Jin Oh et al.
Journal of lipid research, 57(5), 791-808 (2016-03-19)
Endothelial biomechanics is emerging as a key factor in endothelial function. Here, we address the mechanisms of endothelial stiffening induced by oxidized LDL (oxLDL) and investigate the role of oxLDL in lumen formation. We show that oxLDL-induced endothelial stiffening is
Nina Pettersen Hessvik et al.
The Journal of steroid biochemistry and molecular biology, 128(3-5), 154-164 (2011-11-05)
The aim of this study was to explore the effects of 22(S)-hydroxycholesterol (22(S)-HC) on lipid and glucose metabolism in human-derived cells from metabolic active tissues. Docking of T0901317 and 22(S)-HC showed that both substances fitted into the ligand binding domain
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務