推薦產品
品質等級
化驗
98%
形狀
liquid
折射率
n20/D 1.445 (lit.)
bp
159.1 °C (lit.)
密度
1.426 g/mL at 25 °C (lit.)
SMILES 字串
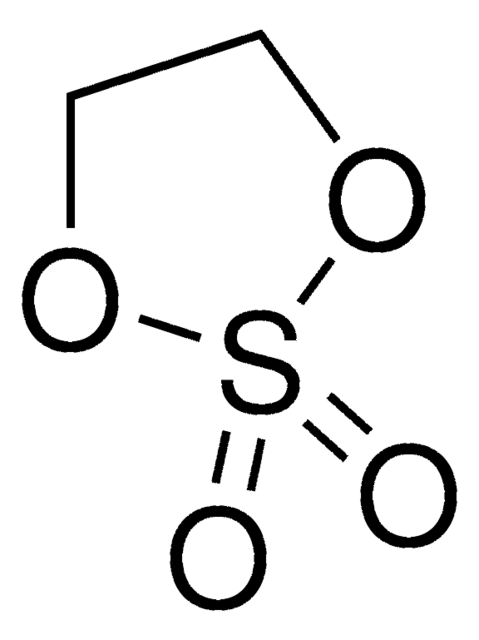
O=S1OCCO1
InChI
1S/C2H4O3S/c3-6-4-1-2-5-6/h1-2H2
InChI 密鑰
WDXYVJKNSMILOQ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
197.1 °F
閃點(°C)
91.7 °C
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Joseph P O'Shea et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 96, 207-216 (2015-07-29)
Novel formulations that overcome the solubility limitations of poorly water soluble drugs (PWSD) are becoming ever more critical to a drug development process inundated with these compounds. There is a clear need for developing bio-enabling formulation approaches to improve oral
Dharmendra K Yadav et al.
AAPS PharmSciTech, 16(4), 855-864 (2015-01-15)
The objective of this study was to develop novel docetaxel phospholipid nanoparticles (NDPNs) for intravenous administration. Modified solvent diffusion-evaporation method was adopted in the NDPN preparation. Central composite design (CCD) was employed in the optimization of the critical formulation factor
Hywel D Williams et al.
Journal of pharmaceutical sciences, 103(6), 1734-1746 (2014-04-18)
The current study determined the extent to which the desorption of lipid-based formulations (LBFs) from a mesoporous magnesium aluminometasilicate (Neusilin®-US2) carrier is governed by drug properties, LBF composition, and LBF-to-adsorbent ratio. A secondary objective was to evaluate the impact of
Hywel D Williams et al.
Journal of pharmaceutical sciences, 103(8), 2441-2455 (2014-07-06)
The Lipid Formulation Classification System Consortium looks to develop standardized in vitro tests and to generate much-needed performance criteria for lipid-based formulations (LBFs). This article highlights the value of performing a second, more stressful digestion test to identify LBFs near
Mette U Anby et al.
Molecular pharmaceutics, 11(11), 4069-4083 (2014-09-30)
The impact of gastrointestinal (GI) processing and first pass metabolism on danazol oral bioavailability (BA) was evaluated after administration of self-emulsifying drug delivery systems (SEDDS) in the rat. Danazol absolute BA was determined following oral and intraduodenal (ID) administration of
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務