推薦產品
化驗
97.5%
mp
132-134 °C (lit.)
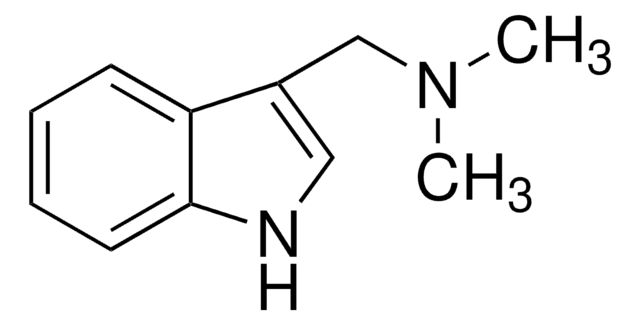
SMILES 字串
CN(C)Cc1c[nH]c2ccccc12
InChI
1S/C11H14N2/c1-13(2)8-9-7-12-11-6-4-3-5-10(9)11/h3-7,12H,8H2,1-2H3
InChI 密鑰
OCDGBSUVYYVKQZ-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
作为反应物用于制备:
- 多巴胺D2受体拮抗剂
- 抗疟疾药
- 5-吲哚基-曼尼希碱
- 增殖抑制剂
- 人肥大细胞糜酶的抑制剂
- DL-色氨酸的制备
- 十字花科植物抗毒素黄铜素的潜在解毒抑制剂
- 3-乙烯基吲哚
- 血清素5-HT6受体配体模板
- 选择性蛋白激酶cδ(PKCδ)下调因子
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
332.6 °F
閃點(°C)
167 °C
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
S Iwata et al.
European journal of pharmacology, 432(1), 63-70 (2001-12-06)
We examined the effects of 2,5,6-tribromo-1-methylgramine (TBG), isolated from bryozoan, and its derivative, 5,6-dibromo-1,2-dimethylgramine (DBG), on the contraction of rat aorta. TBG and DBG decreased the high-K(+)-induced increase in muscle contraction and cytosolic Ca(2+) level ([Ca(2+)](i)), respectively. The inhibitory effects
Brian Chauder et al.
Organic letters, 4(5), 815-817 (2002-03-01)
[reaction: see text] In the presence of NXS (X = Br, I, Cl), gramine derivatives 1, derived by combined directed ortho metalation (DoM)-cross-coupling sequences, rapidly undergo retro-Mannich fragmentation (2) to afford 3-halo indoles 3 in 37-88% yields. A conceptually new
N Nakahata et al.
European journal of pharmacology, 382(2), 129-132 (1999-10-21)
5,6-Dibromo-1,2-dimethylgramine evoked Ca(2+) release from skeletal muscle sarcoplasmic reticulum through ryanodine receptors in a concentration-dependent manner with an EC(50) of 22.2 microM. Since the EC(50) of caffeine was 0.885 mM, 5,6-dibromo-1,2-dimethylgramine was 40 times more sensitive than caffeine. Among 14
E L Barker et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 19(12), 4705-4717 (1999-06-15)
Mutation of a conserved Asp (D98) in the rat serotonin (5HT) transporter (rSERT) to Glu (D98E) led to decreased 5HT transport capacity, diminished coupling to extracellular Na+ and Cl-, and a selective loss of antagonist potencies (cocaine, imipramine, and citalopram
Robert M Williams et al.
Journal of the American Chemical Society, 125(40), 12172-12178 (2003-10-02)
The first total synthesis of paraherquamide A, a potent anthelmintic agent isolated from various Penicillium sp. with promising activity against drug-resistant intestinal parasites, is reported. Key steps in this asymmetric, stereocontrolled total synthesis include a new enantioselective synthesis of alpha-alkylated-beta-hydroxyproline
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務