推薦產品
形狀
powder
SMILES 字串
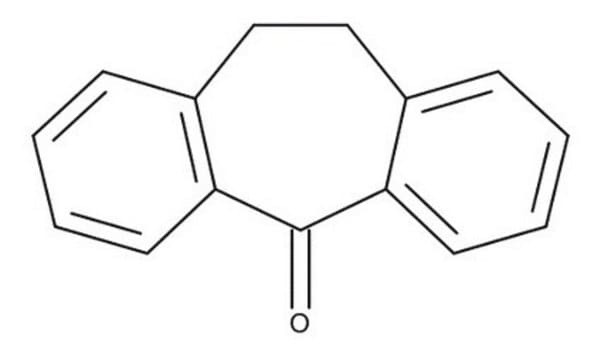
NC(=O)C1c2ccccc2CCc3ccccc13
InChI
1S/C16H15NO/c17-16(18)15-13-7-3-1-5-11(13)9-10-12-6-2-4-8-14(12)15/h1-8,15H,9-10H2,(H2,17,18)
InChI 密鑰
APBVLLORZMAWKI-UHFFFAOYSA-N
應用
Used to study pharmacological activity involved in identification of compounds with central nervous system activity
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Anticonvulsant activities and brain concentrations of cyheptamide and carbamazepine.
G H Wimbish et al.
Proceedings of the Western Pharmacology Society, 23, 75-79 (1980-01-01)
[Determination of plasma lidocaine by gas-liquid chromatography].
I Saavedra et al.
Revista medica de Chile, 115(7), 661-664 (1987-07-01)
G L Jones et al.
Journal of pharmaceutical sciences, 70(6), 618-620 (1981-06-01)
Carbamazepine and cyheptamide have certain stereochemical features in common with phenytoin; when superimposed, two bulky hydrophobic groups in each permit the approximate apposition of two electron donor atoms. The anticonvulsant activity of each compound was determined in mice using a
Josefin Koehn et al.
Antimicrobial agents and chemotherapy, 59(11), 6682-6688 (2015-08-08)
In the treatment of HIV infection, a combination of anti-HIV drugs is commonly used in highly active antiretroviral therapy (HAART). One such combination recommended for clinical therapy consists of the two HIV protease inhibitors atazanavir and ritonavir and the HIV
P W Codding et al.
Journal of medicinal chemistry, 27(5), 649-654 (1984-05-01)
The molecular structures of cyheptamide and 3-hydroxy-3- phenacyloxindole were determined by X-ray diffraction methods. The amide group in both compounds exhibits delocalization of the pi-electrons over the three atoms (N, C, and O), while the bond linking the amide to
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務