推薦產品
種類
(High purity Salts)
品質等級
化驗
≥99.9% trace metals basis
形狀
powder or crystals
solid
雜質
≤1000 ppm (trace metals analysis)
顏色
white to off-white
pH值
≤9.5
mp
53-56 °C (lit.)
溶解度
water: soluble
負離子痕跡
chloride (Cl-): ≤20 ppm
sulfate (SO42-): ≤50 ppm
正離子痕跡
Al: <100 ppm
Cu: <100 ppm
Fe: <100 ppm
K: <100 ppm
Mg: <100 ppm
Na: ≤50 ppm
Pb: <100 ppm
Zn: <100 ppm
應用
battery manufacturing
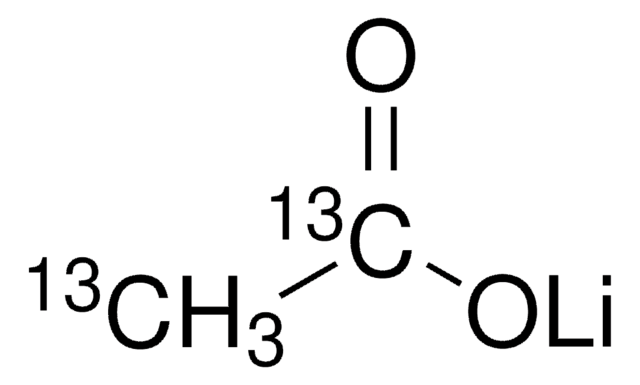
SMILES 字串
[Li+].[H]O[H].[H]O[H].CC([O-])=O
InChI
1S/C2H4O2.Li.2H2O/c1-2(3)4;;;/h1H3,(H,3,4);;2*1H2/q;+1;;/p-1
InChI 密鑰
IAQLJCYTGRMXMA-UHFFFAOYSA-M
尋找類似的產品? 前往 產品比較指南
一般說明
Lithium acetate dihydrate is a soluble white compound with a one-dimensional structure. Lithium acetate dihydrate has various applications in industries such as pharmaceuticals, ceramics, and research laboratories. It is often utilized as a source of lithium ions in chemical reactions and as a precursor in the synthesis of other lithium compounds.
應用
Lithium acetate dihydrate is a significant salt with a wide range of applications. It is utilized as a component in drug formulation and therapy, as a buffer for DNA and RNA gel electrophoresis, and as an additive or catalyst in textiles and polymer production. Additionally, it serves as a ferromagnetic nanoparticle, catalyst, and precursor material for batteries
Our Lithium acetate dihydrate, with a purity of 99.9% on a trace metals basis, serves as an excellent precursor for batteries and catalysis. Its low trace metals content and anions make it particularly well-suited for these applications.
Our Lithium acetate dihydrate, with a purity of 99.9% on a trace metals basis, serves as an excellent precursor for batteries and catalysis. Its low trace metals content and anions make it particularly well-suited for these applications.
- Lithium Iron Pyrophosphate (LiFe1.5P2O7) with monoclinic structures was successfully synthesized using Lithium acetate dihydrate in combination with other metal acetates, in a ratio of Li/Fe/P = 1.05:1.5:2, through a wet-chemical method. Maintaining the appropriate lithium concentration is crucial to prevent stoichiometry loss in the final product. This material has found application as a positive electrode in Lithium-ion batteries. Remarkably, the electrode demonstrates excellent incremental capacity, indicating a stable structure during the initial cycle, with redox peaks observed at 3.33 and 3.22 V versus Li0/Li+
- LiMn2O4 films were synthesized on Au foil using the sol-gel and spin-coating techniques, employing Lithium acetate dihydrate and manganese acetate tetrahydrate in a Li/Mn ratio of 1.1/2. The particles used had an average size of approximately 300 nm. To investigate the morphological changes during over-discharging, the EC-HS-AFM technique was utilized. The images captured revealed the presence of wrinkle-like and step-like structures on the particle surface. These structures were attributed to stresses induced by structural distortion during the phase transformation from cubic (LiMn2O4) to tetragonal (Li2Mn2O4). The formation of the Li2Mn2O4 phase was confirmed through ex situ XRD analysis. Furthermore, by analyzing the EC-HS-AFM images, the particle surface area was quantitatively extracted as a function of potential, providing insights into the irreversible expansion/contraction behavior of the particles
- Cobalt-free cathodes, specifically Mg and Zr modified LiNi0.5Mn1.5O4 (LNMO), were synthesized using Lithium acetate dihydrate and other metal acetates via a citric acid sol-gel method. The modifications aimed to improve the electrochemical performance of the cathode, particularly at high temperatures, by limiting Mn dissolution and adjusting interstitial sites. This modification resulted in increased stability of the cathode, extending the cycle life to 1000 cycles at both 25 and 50 °C
特點和優勢
- Water soluble
- Medium purity (99.9%)
- Low trace metals in ppm level
- Cost effective
- Low Chloride and sulfate levels
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Novel Lithium Iron Pyrophosphate (LiFe1.5P2O7) as a Positive Electrode for Li-Ion Batteries
Ramana C V, et al.
Chemistry of Materials, 19, 5319?5324-5319?5324 (2007)
Operando Imaging of Over-Discharge-Induced Surface Morphology Evolutions of LiMn2O4 Submicron-Sized Particles by Electrochemical High-Speed Atomic Force Microscopy
Yang P, et al.
Langmuir, 39, 13801?13806-13801?13806 (2023)
Evaluation of Electronic?Ionic Transport Properties of a Mg/Zr-Modified LiNi0.5Mn1.5O4 Cathode for Li-Ion Batteries
Balducci L, et al.
ACS Applied Materials & Interfaces, 15, 55620?55632-55620?55632 (2023)
Anhydrous Lithium Acetate Polymorphs and Its Hydrates: Three-Dimensional Coordination Polymers
Casado M F J, et al.
Crystal Growth & Design, 11, 1021-1032 (2011)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務