推薦產品
ligand
thalidomide
品質等級
化驗
≥95.0%
形狀
powder or crystals
儲存溫度
2-8°C
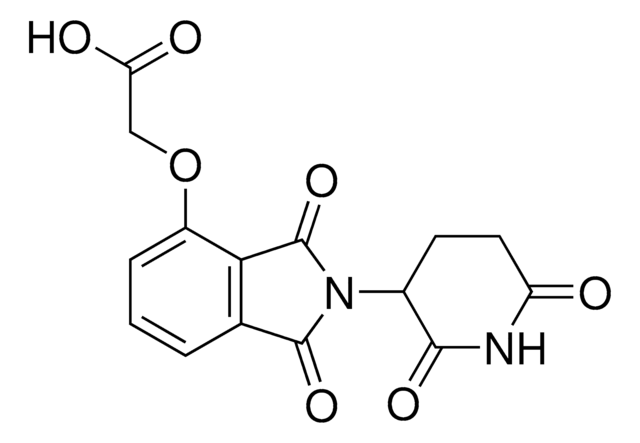
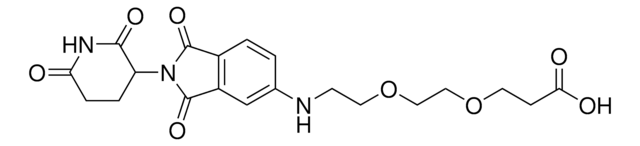
SMILES 字串
O=C(C(F)(F)F)[O-].O=C(COC1=CC=CC(C(N2C3C(NC(CC3)=O)=O)=O)=C1C2=O)NCCCCCCCC[NH3+]
InChI
1S/C23H30N4O6.C2HF3O2/c24-12-5-3-1-2-4-6-13-25-19(29)14-33-17-9-7-8-15-20(17)23(32)27(22(15)31)16-10-11-18(28)26-21(16)30;3-2(4,5)1(6)7/h7-9,16H,1-6,10-14,24H2,(H,25,29)(H,26,28,30);(H,6,7)
InChI 密鑰
AJVLNIUPDHKOPS-UHFFFAOYSA-N
應用
A functionalized cereblon ligand for development of Thalidomide based PROTACs. Allows rapid conjugation with carboxyl linkers due to presence of amine group via peptide coupling reactions. Amenable for linker attachement via reductive amination, and a basic building block for making protein degrader library.
Technology Spotlight:
Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Technology Spotlight:
Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
其他說明
Targeted Protein Degradation by Small Molecules
Destruction of DNA-Binding Proteins by Programmable Oligonucleotide PROTAC (O′PROTAC): Effective Targeting of LEF1 and ERG
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Destruction of DNA-Binding Proteins by Programmable Oligonucleotide PROTAC (O′PROTAC): Effective Targeting of LEF1 and ERG
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
法律資訊
PROTAC® is a registered trademark of Arvinas Operations, Inc., and is used under license.
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Repr. 1A
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
從最近期的版本中選擇一個:
Jingwei Shao et al.
Advanced science (Weinheim, Baden-Wurttemberg, Germany), 8(20), e2102555-e2102555 (2021-08-17)
DNA-binding proteins, including transcription factors (TFs), play essential roles in various cellular processes and pathogenesis of diseases, deeming to be potential therapeutic targets. However, these proteins are generally considered undruggable as they lack an enzymatic catalytic site or a ligand-binding
Daniel P Bondeson et al.
Annual review of pharmacology and toxicology, 57, 107-123 (2016-10-13)
Protein homeostasis networks are highly regulated systems responsible for maintaining the health and productivity of cells. Whereas therapeutics have been developed to disrupt protein homeostasis, more recently identified techniques have been used to repurpose homeostatic networks to effect degradation of
Momar Toure et al.
Angewandte Chemie (International ed. in English), 55(6), 1966-1973 (2016-01-13)
The current inhibitor-based approach to therapeutics has inherent limitations owing to its occupancy-based model: 1) there is a need to maintain high systemic exposure to ensure sufficient in vivo inhibition, 2) high in vivo concentrations bring potential for off-target side effects, and 3) there is
Kedra Cyrus et al.
Molecular bioSystems, 7(2), 359-364 (2010-10-06)
Conventional genetic approaches have provided a powerful tool in the study of proteins. However, these techniques often preclude selective manipulation of temporal and spatial protein functions, which is crucial for the investigation of dynamic cellular processes. To overcome these limitations
Philipp M Cromm et al.
Cell chemical biology, 24(9), 1181-1190 (2017-06-27)
Traditional pharmaceutical drug discovery is almost exclusively focused on directly controlling protein activity to cure diseases. Modulators of protein activity, especially inhibitors, are developed and applied at high concentration to achieve maximal effects. Thereby, reduced bioavailability and off-target effects can
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務