929379
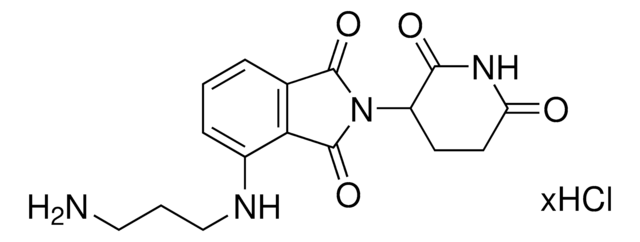
Opto-thalidomide-O-acetamide-C4-NH2 hydrochloride
同義詞:
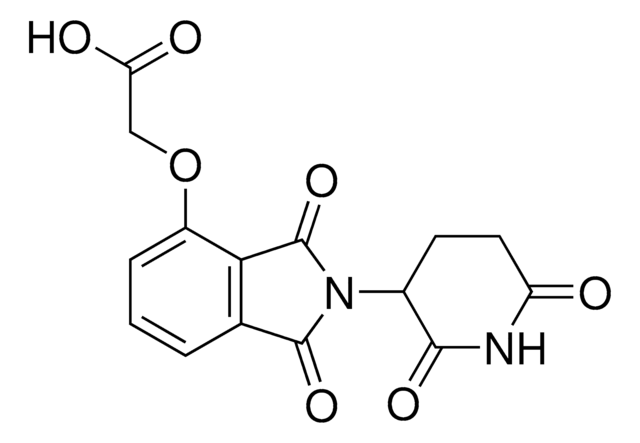
4,5-Dimethoxy-2-nitrobenzyl 3-(4-(2-((4-aminobutyl)amino)-2-oxoethoxy)-1,3-dioxoisoindolin-2-yl)-2,6-dioxopiperidine-1-carboxylate hydrochloride
登入查看組織和合約定價
全部照片(1)
About This Item
經驗公式(希爾表示法):
C29H31N5O12 · xHCl
分子量::
641.58 (free base basis)
分類程式碼代碼:
12352101
NACRES:
NA.22
推薦產品
ligand
thalidomide
品質等級
形狀
powder
官能基
amine
儲存溫度
2-8°C
SMILES 字串
COC1=CC(COC(N2C(CCC(N3C(C4=CC=CC(OCC(NCCCCN)=O)=C4C3=O)=O)C2=O)=O)=O)=C(C=C1OC)[N+]([O-])=O.Cl
應用
Protein degrader building block Opto-thalidomide-O-acetamide-C4-NH2 hydrochloride enables the synthesis of molecules for light-induced targeted protein degradation and PROTAC® (proteolysis-targeting chimeras) research. This conjugate contains a Cereblon (CRBN) recruiting ligand, a rigid linker, and a pendant amine for reactivity with a carboxylic acid on the target ligand. Because even slight alterations in ligands and crosslinkers can affect ternary complex formation between the target, E3 ligase, and degrader, many analogs are prepared to screen for optimal target degradation. When used with other protein degrader building blocks with a terminal amine, parallel synthesis can be used to more quickly generate degrader libraries that feature variation in crosslinker length, composition, and E3 ligase ligand.
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
其他說明
法律資訊
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Repr. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Jing Liu et al.
Science advances, 6(8), eaay5154-eaay5154 (2020-03-05)
By hijacking endogenous E3 ligase to degrade protein targets via the ubiquitin-proteasome system, PROTACs (PRoteolysis TArgeting Chimeras) provide a new strategy to inhibit protein targets that were regarded as undruggable before. However, the catalytic nature of PROTAC potentially leads to
Daniel P Bondeson et al.
Annual review of pharmacology and toxicology, 57, 107-123 (2016-10-13)
Protein homeostasis networks are highly regulated systems responsible for maintaining the health and productivity of cells. Whereas therapeutics have been developed to disrupt protein homeostasis, more recently identified techniques have been used to repurpose homeostatic networks to effect degradation of
Kedra Cyrus et al.
Molecular bioSystems, 7(2), 359-364 (2010-10-06)
Conventional genetic approaches have provided a powerful tool in the study of proteins. However, these techniques often preclude selective manipulation of temporal and spatial protein functions, which is crucial for the investigation of dynamic cellular processes. To overcome these limitations
Philipp M Cromm et al.
Cell chemical biology, 24(9), 1181-1190 (2017-06-27)
Traditional pharmaceutical drug discovery is almost exclusively focused on directly controlling protein activity to cure diseases. Modulators of protein activity, especially inhibitors, are developed and applied at high concentration to achieve maximal effects. Thereby, reduced bioavailability and off-target effects can
Momar Toure et al.
Angewandte Chemie (International ed. in English), 55(6), 1966-1973 (2016-01-13)
The current inhibitor-based approach to therapeutics has inherent limitations owing to its occupancy-based model: 1) there is a need to maintain high systemic exposure to ensure sufficient in vivo inhibition, 2) high in vivo concentrations bring potential for off-target side effects, and 3) there is
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務
![3-[1,3-Dihydro-4-(5-hydroxy-1-pentyn-1-yl)-1-oxo-2H-isoindol-2-yl]-2,6-piperidinedione ≥95.0%](/deepweb/assets/sigmaaldrich/product/structures/165/184/ebc29f1b-f63f-4e48-afb5-b3aa4c69795a/640/ebc29f1b-f63f-4e48-afb5-b3aa4c69795a.png)