917427
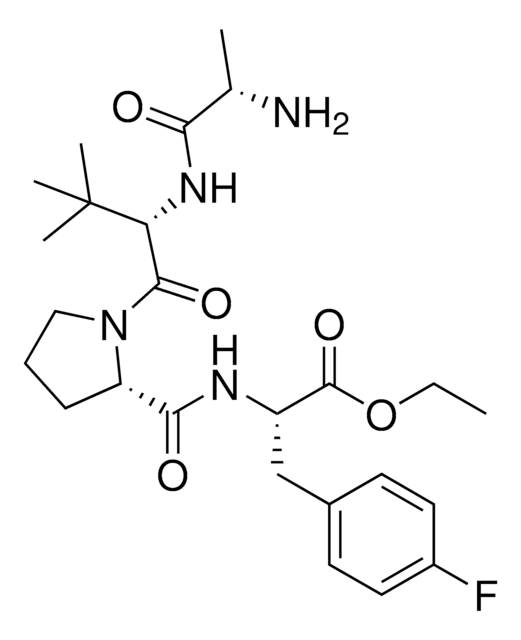
A1V1PF2-OEt-C6-NH2 hydrochloride
同義詞:
AVP conjugate for IAP-mediated protein degrader development, Ethyl (S)-2-((S)-1-((S)-2-((S)-2-((6-aminohexyl)amino)propanamido)-3,3-dimethylbutanoyl)pyrrolidine-2-carboxamido)-3-(4-fluorophenyl)propanoate hydrochloride, SNIPER building block
About This Item
推薦產品
ligand
A1V1PF2
品質等級
形狀
powder
反應適用性
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
官能基
amine
儲存溫度
2-8°C
SMILES 字串
C[C@H](NCCCCCCN)C(N[C@H](C(N1CCC[C@H]1C(N[C@H](C(OCC)=O)CC2=CC=C(C=C2)F)=O)=O)C(C)(C)C)=O.Cl
應用
Building blocks in this series:
917710 A1V1PF2-OEt
917427 A1V1PF2-OEt-C6-NH2 hydrochloride
917672 A1V1PF2-OEt-C10-NH2 hydrochloride
917923 A1V1PF2-OEt-PEG1-NH2 hydrochloride
916676 A1V1PF2-OEt-PEG3-NH2 hydrochloride
Technology Spotlight: Degrader Building Blocks with Inhibitor of Apoptosis Protein (IAP) In Silico-Derived Ligands
其他說明
法律資訊
相關產品
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
文章
Plate of 80 ligands against E3 ligase IAP designed by ComInnex; allows creation of bifunctional targeted protein degraders or molecular glues.
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Protein Degrader Building Blocks 是一系列交聯劑-E3 配體結合物,具有懸垂功能基團,可與目標配體共價連結。
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務