917184
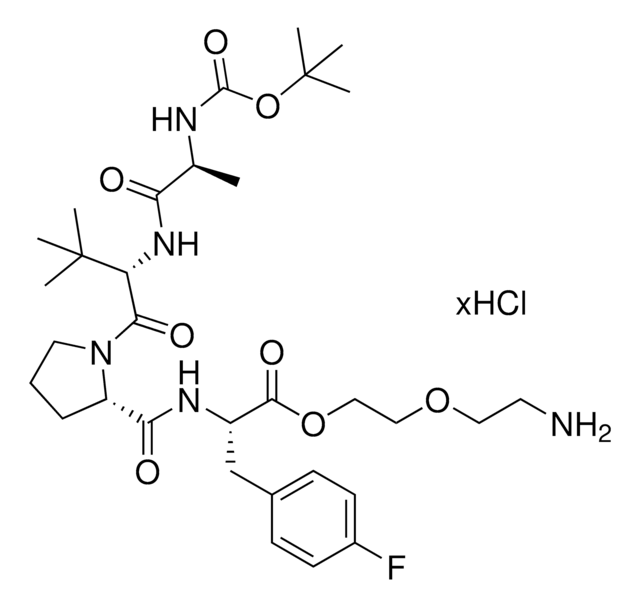
BocA1V1PF2-OC10-NH2 hydrochloride
同義詞:
10-Aminodecyl (S)-2-((S)-1-((S)-2-((S)-2-((tert-butoxycarbonyl)amino)propanamido)-3,3-dimethylbutanoyl)pyrrolidine-2-carboxamido)-3-(4-fluorophenyl)propanoate hydrochloride, AVP conjugate for IAP-mediated protein degrader development, SNIPER building block
About This Item
推薦產品
ligand
BocA1V1PF2
品質等級
形狀
powder
反應適用性
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
官能基
amine
儲存溫度
2-8°C
SMILES 字串
C[C@H](NC(OC(C)(C)C)=O)C(N[C@H](C(N1CCC[C@H]1C(N[C@H](C(OCCCCCCCCCCN)=O)CC2=CC=C(C=C2)F)=O)=O)C(C)(C)C)=O.Cl
應用
Developed in partnership with ComInnex, this conjugate contains an in silico-derived IAP-recruiting ligand, an alkyl-chain crosslinker, and a pendant amine for reactivity with an acid on a target warhead. Because even slight alterations in ligands and crosslinkers can affect ternary complex formation between the target, E3 ligase, and protein degrader, many analogs are prepared to screen for optimal target degradation. When used with other When used with other protein degrader building blocks with a terminal amine, including CRBN and VHL targeted, parallel synthesis can be used to more quickly generate SNIPER and PROTAC® degrader libraries that feature variation in crosslinker length, composition, and E3 ligase ligand. Learn more about the novel IAP ligands generated through virtual screening of AVP mimetics in our Technology Spotlight.
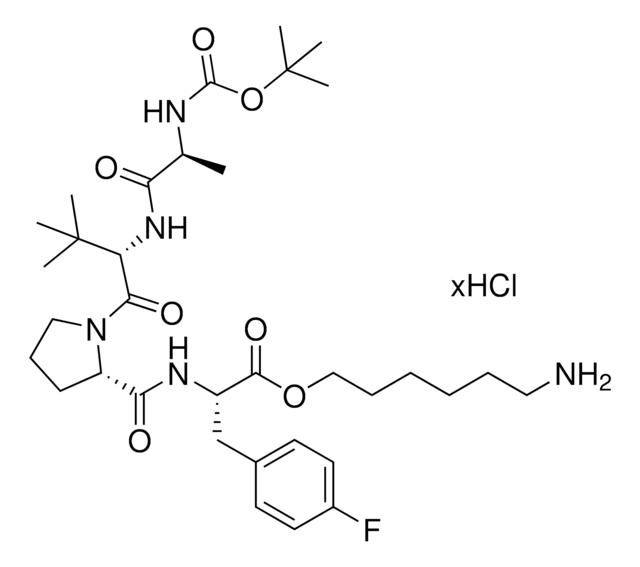
Building blocks in this series:
917478 BocA1V1PF2
916927 BocA1V1PF2-OC6-NH2 hydrochloride
917184 BocA1V1PF2-OC10-NH2 hydrochloride
917435 BocA1V1PF2-OPEG1-NH2 hydrochloride
917680 BocA1V1PF2-OPEG3-NH2 hydrochloride
其他說明
法律資訊
相關產品
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
文章
Plate of 80 ligands against E3 ligase IAP designed by ComInnex; allows creation of bifunctional targeted protein degraders or molecular glues.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務