推薦產品
一般說明
keYPhos™is an ylide-functionalized phosphine ligand developed in the lab of Prof. V. Gessner at the Ruhr-University Bochum with demonstrated uses in Pd-catalyzed cross coupling reactions, including the arylation of ketones and arylation of amines. keYPhos™ is part of the YPhos™ family of ligands, also containing the joYPhos™ and trYPhos™ ligands.
應用
The electron-rich and sterically demanding keYPhos™ has a methyl group in the ylide-backbone and is a valuable ligand for the palladium catalyzed coupling of aryl chlorides with primary and secondary alkyl and aryl amines at room temperature. keYPhos™has been used in the gold(I)-catalyzed hydroamination of acetylene, and has shown to be effective in a range of Buchwald-Hartwig amination reactions. The strong electron-donor strength and sterically demanding nature of the ligand has been shown to increase the rate of formation of the catalytically active mono-phosphine palladium species, often leading to decreased reaction times or allowing the use of lower reaction temperatures.
Learn more about ylide-functionalized phosphines (YPhos)
Learn more about ylide-functionalized phosphines (YPhos)
特點和優勢
Advantages of the keYPhos™ligand over less electron rich ligand sources include, increased substrate scope in Buchwald-Hartwig amination reactions, including aryl chlorides, the use of more mild reaction conditions and improved activity in in C-N and C-C cross coupling reactions. keYPhos™ has been shown to perform well with common palladium sources such as Pd2(dba)3, Pd(OAc)2, [Pd(allyl)Cl]2 or [Pd(cinamyl)Cl]2.
法律資訊
Product of Umicore
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at www.pmc.umicore.com.
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at www.pmc.umicore.com.
Yphos is a trademark of Umicore AG & Co. KG
相關產品
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Jens Tappen et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 26(19), 4281-4288 (2020-01-24)
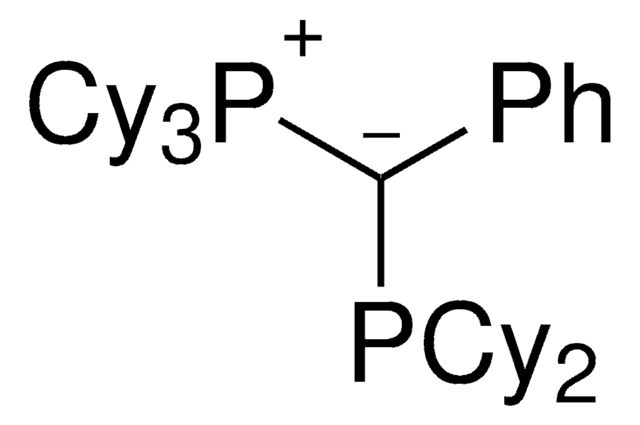
Palladium allyl, cinnamyl, and indenyl complexes with the ylide-substituted phosphines Cy3 P+ -C- (R)PCy2 (with R=Me (L1) or Ph (L2)) and Cy3 P+ -C- (Me)PtBu2 (L3) were prepared and applied as defined precatalysts in C-N coupling reactions. The complexes are
Sébastien Lapointe et al.
Accounts of chemical research, 55(5), 770-782 (2022-02-17)
The development of homogeneous catalysts is strongly connected to the design of new, sophisticated ligands, which resolve limitations of a given reaction protocol by manipulating the electronic properties of the metal and its spatial environment. Phosphines are a privileged class
Formation of exceptional monomeric YPhos-PdCl2 complexes with high activities in coupling reactions.
Ilja Rodstein et al.
Chemical science, 13(45), 13552-13562 (2022-12-13)
The use of well-defined palladium(ii) complexes as precatalysts for C-X cross-coupling reactions has improved the use of palladium catalysts in organic synthesis including large-scale processes. Whereas sophisticated Pd(ii) precursors have been developed in the past years to facilitate catalyst activation
Xiao-Qiang Hu et al.
Organic letters, 21(18), 7558-7562 (2019-08-31)
Ylide-functionalized phosphine (YPhos) ligands allow the palladium-catalyzed α-arylation of alkyl ketones with aryl chlorides with record setting activity. Using a cyclohexyl-substituted YPhos ligand, a wide range of challenging ketone substrates was efficiently and selectively monoarylated under mild conditions. A newly
Ilja Rodstein et al.
The Journal of organic chemistry, 85(22), 14674-14683 (2020-09-11)
Ylide-substituted phosphines have been shown to be excellent ligands for C-N coupling reactions under mild reaction conditions. Here we report studies on the impact of the steric demand of the substituent in the ylide-backbone on the catalytic activity. Two new
文章
Explore innovative palladium-catalyzed coupling reactions with ylide-substituted phosphines. Learn about their impressive capabilities, enabling milder conditions and access to aryl chlorides.
Electron-rich ylide-substituted phosphine ligands allow for palladium catalyzed coupling reactions at mild reaction conditions. These YPhos ligands enable the conversion of aryl chlorides with short reaction times.
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務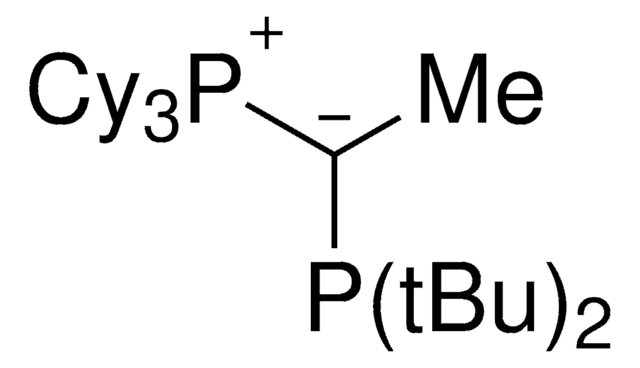
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)






![Dichloro[2,2′-bis(diphenylphosphino)-1,1′-binaphthyl]palladium(II) 97%](/deepweb/assets/sigmaaldrich/product/structures/351/904/a38f7a0d-b8a0-448f-b949-4b983e7f1eb3/640/a38f7a0d-b8a0-448f-b949-4b983e7f1eb3.png)