912131
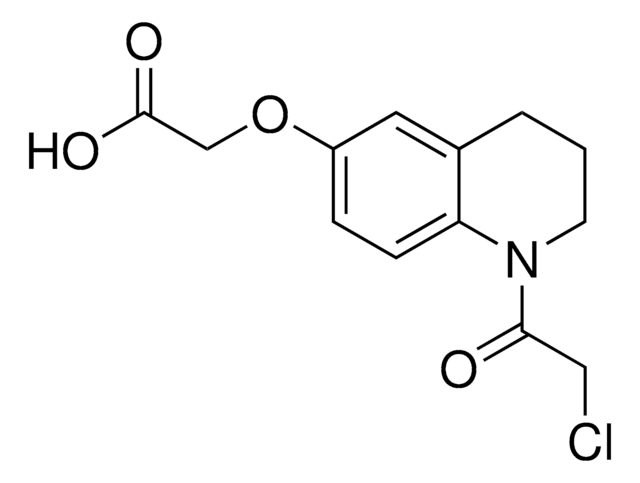
2-Chloro-1-(6-methoxy-1,2,3,4-tetrahydroquinolin-1-yl)ethan-1-one
≥95%
同義詞:
1-(Chloroacetyl)-1,2,3,4-tetrahydro-6-quinolinyl methyl ether, 2-Chloro-1-(6-methoxy-3,4-dihydroquinolin-1(2H)-yl)ethan-1-one, Electrophilic scout fragment, KB02, Scout fragment for targetable cysteine
登入查看組織和合約定價
全部照片(2)
About This Item
暫時無法取得訂價和供貨情況
推薦產品
品質等級
化驗
≥95%
形狀
chunks
儲存溫度
2-8°C
SMILES 字串
ClCC(=O)N1CCCc2c1ccc(c2)OC
InChI
1S/C12H14ClNO2/c1-16-10-4-5-11-9(7-10)3-2-6-14(11)12(15)8-13/h4-5,7H,2-3,6,8H2,1H3
InChI 密鑰
XJPUWRWIBSSPSL-UHFFFAOYSA-N
應用
2-Chloro-1-(6-methoxy-1,2,3,4-tetrahydroquinolin-1-yl)ethan-1-one is a cysteine-reactive small-molecule fragment for chemoproteomic and ligandability studies for both traditionally druggable proteins as well as "undruggable," or difficult-to-target, proteins. This fragment electrophile, or "scout" fragment, can be used alone in fragment-based covalent ligand discovery or incorporated into bifunctional tools such as electrophilic PROTAC® molecules for targeted protein degradation as demonstrated by the Cravatt Lab Lab for E3 ligase discovery.
其他說明
法律資訊
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
相關產品
產品號碼
描述
訂價
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Kristine Senkane et al.
Angewandte Chemie (International ed. in English), 58(33), 11385-11389 (2019-06-22)
Reversible covalency, achieved with, for instance, highly electron-deficient olefins, offers a compelling strategy to design chemical probes and drugs that benefit from the sustained target engagement afforded by irreversible compounds, while avoiding permanent protein modification. Reversible covalency has mainly been
Xiaoyu Zhang et al.
Nature chemical biology, 15(7), 737-746 (2019-06-19)
Ligand-dependent protein degradation has emerged as a compelling strategy to pharmacologically control the protein content of cells. So far, however, only a limited number of E3 ligases have been found to support this process. Here, we use a chemical proteomic
Keriann M Backus et al.
Nature, 534(7608), 570-574 (2016-06-17)
Small molecules are powerful tools for investigating protein function and can serve as leads for new therapeutics. Most human proteins, however, lack small-molecule ligands, and entire protein classes are considered 'undruggable'. Fragment-based ligand discovery can identify small-molecule probes for proteins
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務