推薦產品
形狀
solid
品質等級
mp
40-46 °C
官能基
amine
fluoro
儲存溫度
−20°C
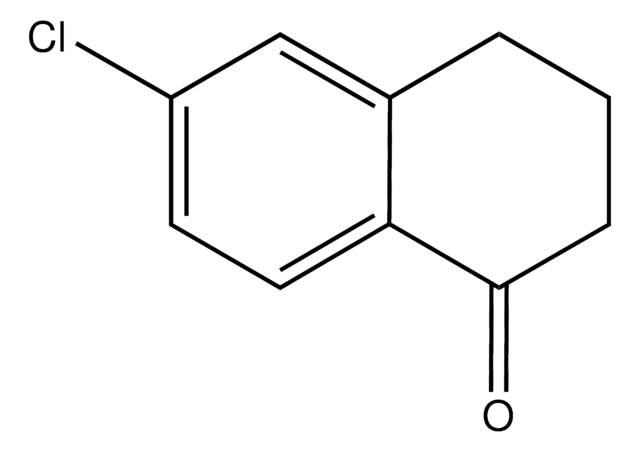
SMILES 字串
FC(F)(F)c1cc(cc(c1)C(=O)N(SC(=S)OCC)C(C)(C)C)C(F)(F)F
InChI 密鑰
QDCXZVWZLHPTDJ-UHFFFAOYSA-N
應用
As reported by the Alexanian. laboratory, this reagent is used for site-selective, intermolecular C-H xanthylation of alkanes, leading to the rapid diversification of otherwise inert C-H bonds. Once installed, the xanthate group provides direct access to diverse product analogues via several aliphatic C-H transformations, including halogenation, deuteration, vinylation, and hydroxylation. Product is best stored in foil-wrapped vials in the freezer when not in use; however, it can be weighed out on the bench without risk of decomposition. This product is offered collaboratively with UNC-Chapel Hill and Erik Alexanian.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
William L Czaplyski et al.
Journal of the American Chemical Society (2016-10-16)
Intermolecular functionalizations of aliphatic C-H bonds offer unique strategies for the synthesis and late-stage derivatization of complex molecules, but the chemical space accessible remains limited. Herein, we report a transformation significantly expanding the chemotypes accessible via C-H functionalization. The C-H
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務