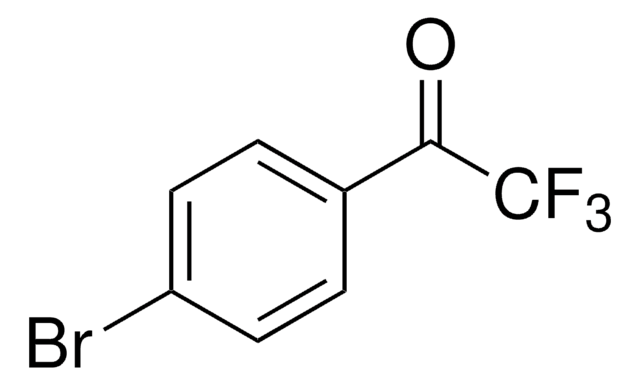
推薦產品
化驗
95%
形狀
liquid
反應適用性
reaction type: C-C Bond Formation
折射率
n20/D 1.4954
n20/D 1.497 (lit.)
bp
94-96 °C/35 mmHg (lit.)
密度
1.293 g/mL at 25 °C (lit.)
1.3178 g/mL at 25 °C
官能基
chloro
fluoro
ketone
phenyl
SMILES 字串
FC(F)(Cl)C(=O)c1ccccc1
InChI
1S/C8H5ClF2O/c9-8(10,11)7(12)6-4-2-1-3-5-6/h1-5H
InChI 密鑰
MNOONJNILVDLSW-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
2-Chloro-2,2-difluoroacetophenone is a difluorocarbene reagent, which is generally used in the synthesis of 2,2-difluoro enol silyl ethers, and gem -difluoromethene derived compounds.[1]
應用
2-Chloro-2,2-difluoroacetophenone can be used:
- As a reagent in the difluoromethylation of various phenols to yield aryl difluoromethyl ethers.[2]
- As a precursor in the Baylis-Hillman reaction of fluoroalkyl ketones to obtain chlorodifluoromethyl containing products.[3]
- As a substrate in the synthesis of propargyl alcohols using a novel ruthenium catalyst.[4]
Reagent is an effective product for the synthesis of difluoromethylated phenols in the presence of mild base and aqueous solvent.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
160.0 °F
閃點(°C)
71.1 °C
Octahedral ruthenium complex with exclusive metal-centered chirality for highly effective asymmetric catalysis
Zheng Y, et al.
Journal of the American Chemical Society, 139(12), 4322-4325 (2017)
2-Chloro-2,2-difluoroacetophenone
Encyclopedia of Reagents for Organic Synthesis, Second Edition (2009)
Study of Fluorocarbonyls for the Baylis- Hillman Reaction
Ram Reddy MV, et al.
The Journal of Organic Chemistry, 67(15), 5382-5385 (2002)
Laijun Zhang et al.
The Journal of organic chemistry, 71(26), 9845-9848 (2006-12-16)
A novel and non-ODS-based (ODS = ozone-depleting substance) preparation of 2-chloro-2,2-difluoroacetophenone (1) was achieved in high yield by using 2,2,2-trifluoroacetophenone as the starting material. Compound 1 was found to act as a good difluorocarbene reagent, which readily reacts with a
2-Chloro-2, 2-difluoroacetophenone: a non-ODS-based difluorocarbene precursor and its use in the difluoromethylation of phenol derivatives
Zhang L, et al.
The Journal of Organic Chemistry, 71(26), 9845-9848 (2006)
相關內容
Prof. Jinbo Hu's lab focuses on developing new fluorination reagents and reactions, including difluoromethylation and monofluoromethylation.
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務