全部照片(1)
About This Item
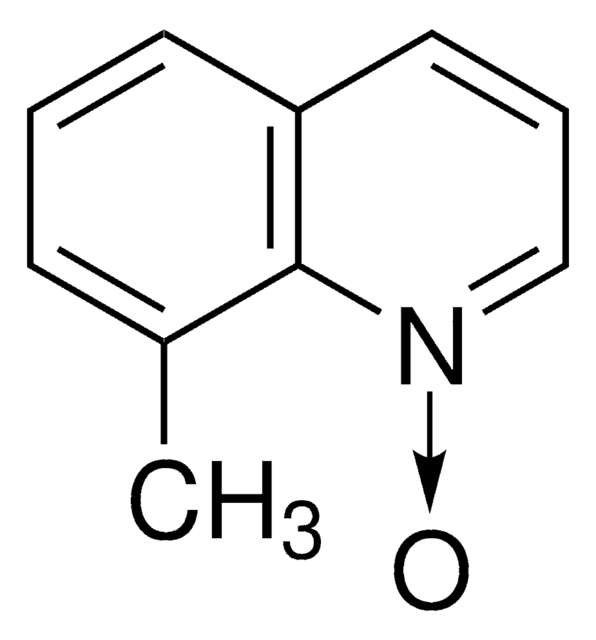
經驗公式(希爾表示法):
C12H13NO
CAS號碼:
分子量::
187.24
MDL號碼:
分類程式碼代碼:
12352005
PubChem物質ID:
NACRES:
NA.22
暫時無法取得訂價和供貨情況
推薦產品
化驗
97%
形狀
liquid
折射率
n20/D 1.644
密度
1.134 g/mL at 25 °C
儲存溫度
2-8°C
SMILES 字串
CC(C)c1cccc2ccc[n+]([O-])c12
InChI
1S/C12H13NO/c1-9(2)11-7-3-5-10-6-4-8-13(14)12(10)11/h3-9H,1-2H3
InChI 密鑰
LYLSTLGFFOQSKE-UHFFFAOYSA-N
應用
8-Isopropylquinoline N-oxide can be used as a reagent:
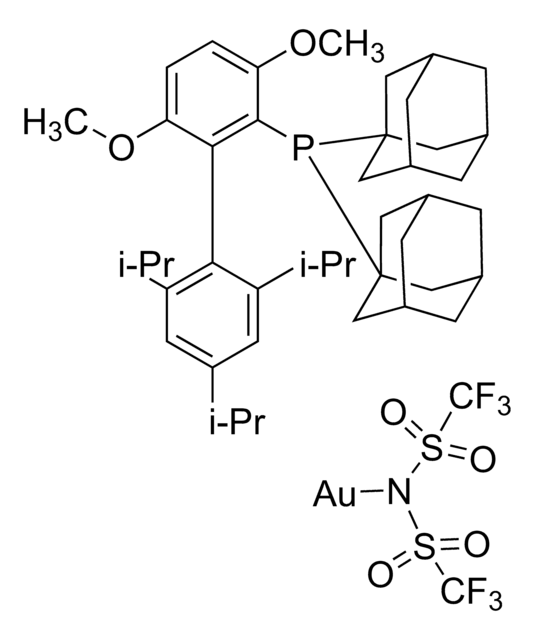
- In the oxidative cyclization of diynes in the presence of gold catalyst.[1]
- For the preparation of pyrrolo[3,4-c]quinolin-1-ones by asymmetric alkyne oxidation of chiral N-propargyl ynamides in the presence of a copper catalyst.[2]
- In the synthesis of 8-(1-methylethyl)-2-[(4-methylphenyl)sulfonyl]- quinoline by deoxygenative and selective sulfonylation with sodium p-toluenesulfinate using iodine/TBHP as a catalyst.[3]
儲存類別代碼
12 - Non Combustible Liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Iodine/TBHP-Promoted One-Pot Deoxygenation and Direct 2-Sulfonylation of Quinoline N-Oxides with Sodium Sulfinates: Facile and Regioselective Synthesis of 2-Sulfonylquinolines
Sumunnee L, et al.
European Journal of Organic Chemistry, 2017(5), 1025-1032 (2017)
Recent advances in catalytic asymmetric intermolecular oxidation of alkynes
Shen W-B and Tang X-T
Organic & Biomolecular Chemistry, 17(30), 7106-7113 (2019)
Pascal Nösel et al.
Journal of the American Chemical Society, 135(41), 15662-15666 (2013-09-21)
In the presence of a gold catalyst an unprecedented oxidative cyclization of diynes takes place. The reaction cascade is initiated by an oxygen transfer from a N-oxide onto a gold-activated alkyne. The formed α-oxo carbene is transferred across the second
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務