全部照片(1)
About This Item
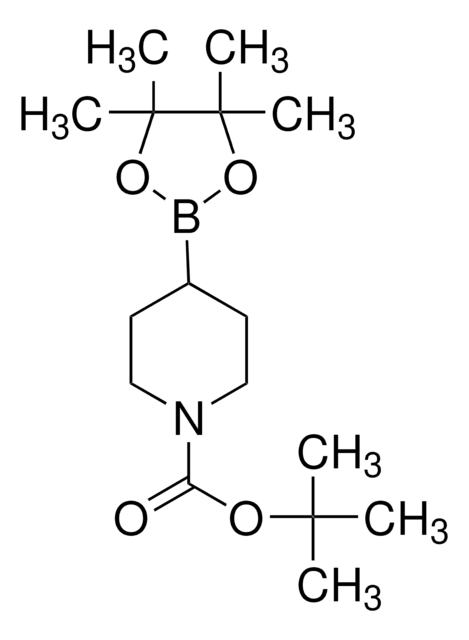
經驗公式(希爾表示法):
C16H28BNO4
CAS號碼:
分子量::
309.21
MDL號碼:
分類程式碼代碼:
12352103
PubChem物質ID:
NACRES:
NA.22
暫時無法取得訂價和供貨情況
推薦產品
品質等級
化驗
95%
形狀
powder
mp
100-114 °C
SMILES 字串
CC(C)(C)OC(=O)N1CCC(=CC1)B2OC(C)(C)C(C)(C)O2
InChI
1S/C16H28BNO4/c1-14(2,3)20-13(19)18-10-8-12(9-11-18)17-21-15(4,5)16(6,7)22-17/h8H,9-11H2,1-7H3
InChI 密鑰
VVDCRJGWILREQH-UHFFFAOYSA-N
一般說明
應用
Reagent used for
Reagent used in Preparation of several enzymatic inhibitors and receptor ligands
- Suzuki-Miyaura cross-coupling using palladium phosphine catalyst[1]
- Palladium-catalyzed ligand-controlled regioselective Suzuki coupling[2]
- Palladium-catalyzed Suzuki-Miyaura coupling[3]
- Suzuki coupling followed by iodolactonization reaction[4]
- Wrenchnolol derivative optimized for gene activation in cells[5]
Reagent used in Preparation of several enzymatic inhibitors and receptor ligands
- Orally active anaplastic lymphoma kinase inhibitors[6]
- Oxazolecarboxamides as diacylglycerol acyltransferase-1 inhibitors for treatment of obesity and diabetes[7]
- 4-arylpiperidinyl amides and N-arylpiperidin-3-yl-cyclopropanecarboxamides as novel melatonin receptor ligands[8]
- Quinazoline analogs as glucocerebrosidase inhibitors with chaperone activity for treatment of Gaucher disease, a lysosomal storage disorder[9]
- Arylpiperazine and piperidine ethers as dual acting norepinephrine reuptake inhibitors and 5-HT1A partial agonists[10]
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
Design and synthesis of 4-arylpiperidinyl amide and N-arylpiperidin-3-yl-cyclopropanecarboxamide derivatives as novel melatonin receptor ligands
Li, G.; et al.
Bioorganic & Medicinal Chemistry, 21, 1236-1242 (2011)
Paul M Wehn et al.
Organic letters, 11(24), 5666-5669 (2009-12-17)
A novel approach to the synthesis of substituted 5-amino- and 3-amino-1,2,4-thiadiazoles beginning from a common precursor has been achieved. Derivatization by palladium-catalyzed Suzuki-Miyaura coupling enables the rapid preparation of analogs around this pharmaceutically relevant core. FMO calculations rationalize the observed
Preparation of 3,4-fused-spiro[furan-5(5H),4'-piperidin]-2-one
Liu, J.; et al.
Tetrahedron Letters, 50, 5228-5230 (2009)
Kazutomo Kinoshita et al.
Bioorganic & medicinal chemistry, 20(3), 1271-1280 (2012-01-10)
Anaplastic lymphoma kinase (ALK) receptor tyrosine kinase is considered an attractive therapeutic target for human cancers, especially non-small cell lung cancer (NSCLC). Our previous study revealed that 8,9-side-chains of 6,6-dimethyl-11-oxo-6,11-dihydro-5H-benzo[b]carbazole scaffold crucially affected kinase selectivity, cellular activity, and metabolic stability.
Discovery and pharmacological characterization of aryl piperazine and piperidine ethers as dual acting norepinephrine reuptake inhibitors and 5-HT1A partial agonists
Gray, D. L.; et al.
Bioorganic & Medicinal Chemistry, 19, 6604-6607 (2009)
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務





![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

