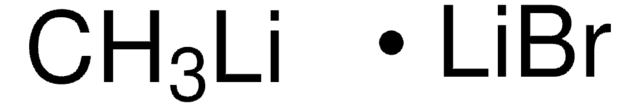推薦產品
形狀
liquid
反應適用性
reagent type: reductant
濃度
1 M in THF
密度
0.905 g/mL at 25 °C
SMILES 字串
[Na+].[AlH4-]
InChI
1S/Al.Na.4H/q-1;+1;;;;
InChI 密鑰
MNYCIYRTKITUKR-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Reaction kinetics of sodium aluminum hydrides may be improved by mechanical grinding or chemical modification. This may result in achieving reversible hydrogen storage at lower temperatures (80-140oC). It may behave as a reducing agent. Reaction kinetics of graphite and Ti-doped NaAlH4 was investigated. Presence of Ti doping enhances the kinetics of absorption and desorption. TiO2, CeO2, La2O3, Pr2O3, Nd2O3, Sm2O3, Eu2O3 and Gd2O3 metal oxides were studied for their catalytic behavior in improving the storage kinetics of NAlH4.
應用
NaAlH4 may be used in the deoxygenative dimerization of carbonyls and alcohols and in hydroalumination of alkenes and alkynes.
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Carc. 2 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3 - Water-react 2
標靶器官
Respiratory system
安全危害
儲存類別代碼
4.3 - Hazardous materials which set free flammable gases upon contact with water
水污染物質分類(WGK)
WGK 1
閃點(°F)
-7.6 °F - closed cup
閃點(°C)
-22 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Studies on metal oxide nanoparticles catalyzed sodium aluminum hydride.
Pukazhselvan D
Energy, 35(12), 5037-5042 (2010)
Effect of graphite as a co-dopant on the dehydrogenation and hydrogenation kinetics of Ti-doped sodium aluminum hydride
Wang J,et al.
J. Alloy Compounds, 395(1-2), 252-262 (2005)
Titanium?halide catalyst-precursors in sodium aluminum hydrides.
Majzoub EH and Gross KJ
J. Alloy Compounds, 356, 363-367 (2003)
Gugelchuk M, et al. et al.
e-EROS Encyclopedia of Reagents for Organic Synthesis. (2009)
Sodium alanates for reversible hydrogen storage,
Zaluska A, et al.
J. Alloy Compounds, 298(1-2), 125-134 (2000)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務