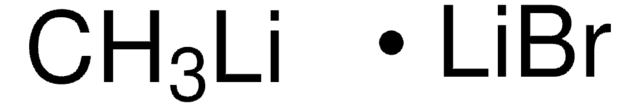推薦產品
化驗
≥94%
形狀
solid
光學活性
[α]20/D +94°, c = 0.5 in chloroform
SMILES 字串
COc1ccc2ccccc2c1-c3c(ccc4ccccc34)P(c5ccccc5)c6ccccc6
InChI
1S/C33H25OP/c1-34-30-22-20-24-12-8-10-18-28(24)32(30)33-29-19-11-9-13-25(29)21-23-31(33)35(26-14-4-2-5-15-26)27-16-6-3-7-17-27/h2-23H,1H3
InChI 密鑰
KRWTWSSMURUMDE-UHFFFAOYSA-N
一般說明
R-MOP是以双萘为主链的膦配体。
應用
法律資訊
与 Takasago 联合销售,仅供研究之用。US5231202
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
T Hayashi
Accounts of chemical research, 33(6), 354-362 (2000-07-13)
Chiral monophosphines, whose chirality is due to biaryl axial chirality, have been prepared from enantiomerically pure 2, 2'-dihydroxy-1,1'-binaphthyl and demonstrated to be highly efficient chiral ligands for transition-metal-catalyzed organic transformations, especially for reactions where chelating bisphosphine ligands cannot be used.
Gaku Hattori et al.
Journal of the American Chemical Society, 132(30), 10592-10608 (2010-07-14)
The scope and limitations of the copper-catalyzed propargylic amination of various propargylic esters with amines are presented, where optically active diphosphines such as Cl-MeO-BIPHEP and BINAP work as good chiral ligands. A variety of secondary amines are available as nucleophiles
The synthesis of P-stereogenic MOP analogues and their use in rhodium catalyzed asymmetric addition
Clarke, E. F.; et al.
Journal of Organometallic Chemistry, 696, 3608-3615 (2011)
Rhodium-catalyzed asymmetric addition of aryl- and alkenylboronic acids to isatins.
Ryo Shintani et al.
Angewandte Chemie (International ed. in English), 45(20), 3353-3356 (2006-04-06)
Uozomi, Y.
Journal of the American Chemical Society, 9887-9887 (1991)
文章
Chiral diene ligands enable asymmetric transformations, constructing enantioenriched compounds from achiral substrates efficiently.
Hydrogenation, Asymmetric Catalysis, Binap, SEGPHOS®, Aldol reaction, Alkenylation, Arylation, Mannich reaction, Fluorination, Michael addition, Hydrosilylation, Cycloaddition, Takasago
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
![[1,1′-双(二-叔丁基膦基)二茂铁]二氯合钯(II) 98%](/deepweb/assets/sigmaaldrich/product/structures/192/459/02d1239c-1119-49d9-b392-a04d8f53855c/640/02d1239c-1119-49d9-b392-a04d8f53855c.png)
![(S)-(+)-(3,5-二氧-4-磷-环庚并[2,1-a;3,4- a′]二萘-4-基)二甲胺 97%](/deepweb/assets/sigmaaldrich/product/structures/400/008/628143de-3954-440a-ba9c-4c0ff8e44663/640/628143de-3954-440a-ba9c-4c0ff8e44663.png)

![(S)-(+)-N-(3,5-Dioxa-4-phosphacyclohepta[2,1-a;3,4-a′]dinaphthalen-4-yl)-dibenzo[b,f]azepine ≥95% (elemental analysis)](/deepweb/assets/sigmaaldrich/product/structures/575/489/d54360f9-5a59-43f2-bc44-42f5fa92b588/640/d54360f9-5a59-43f2-bc44-42f5fa92b588.png)

![(R)-(–)-4,12-双(二苯基膦)-[2.2]-对环芳烷 96%](/deepweb/assets/sigmaaldrich/product/structures/131/143/7e18cd49-a90e-4d89-a189-4f37ad9e6cd2/640/7e18cd49-a90e-4d89-a189-4f37ad9e6cd2.png)