全部照片(1)
About This Item
經驗公式(希爾表示法):
C42H34O8P2Ru
CAS號碼:
分子量::
829.73
MDL號碼:
分類程式碼代碼:
12352005
PubChem物質ID:
NACRES:
NA.22
推薦產品
形狀
powder
儲存溫度
2-8°C
InChI
1S/C38H28O4P2.2C2H4O2.Ru/c1-5-13-27(14-6-1)43(28-15-7-2-8-16-28)33-23-21-31-37(41-25-39-31)35(33)36-34(24-22-32-38(36)42-26-40-32)44(29-17-9-3-10-18-29)30-19-11-4-12-20-30;2*1-2(3)4;/h1-24H,25-26H2;2*1H3,(H,3,4);/q;;;+2/p-2
InChI 密鑰
BHGLLIGZFQVMBJ-UHFFFAOYSA-L
應用
(S)-Ru(OAc)2(SEGPHOS®) can be used as a catalyst:
- To prepare highly chemo, enantio, and diastereoselective primary β-amino lactams by asymmetric reductive amination of racemic β-keto lactams.
- To synthesize chiral primary diarylmethylamines and sterically bulky benzylamines from diaryl and sterically hindered ketones via asymmetric reductive amination reaction.
- For the conversion of levulinic acid to optically active γ-valerolactone via asymmetric hydrogenation reaction.
法律資訊
与 Takasago 合作销售,仅用于研究目的。日本注册号 3148136
SEGPHOS is a registered trademark of Takasago Intl. Corp.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Direct asymmetric reduction of levulinic acid to gamma-valerolactone: synthesis of a chiral platform molecule
Tukacs JM, et al.
Green Chemistry, 17(12), 5189-5195 (2015)
Dynamic Kinetic Asymmetric Reductive Amination: Synthesis of Chiral Primary ?-Amino Lactams
Lou Y, et al.
Angewandte Chemie (International ed. in English), 57(43), 14193-14197 (2018)
Ruthenium-Catalyzed Direct Asymmetric Reductive Amination of Diaryl and Sterically Hindered Ketones with Ammonium Salts and H2
Hu L, et al.
Angewandte Chemie (International Edition in English), 132(13), 5359-5363 (2020)
文章
Hydrogenation, Asymmetric Catalysis, Binap, SEGPHOS®, Aldol reaction, Alkenylation, Arylation, Mannich reaction, Fluorination, Michael addition, Hydrosilylation, Cycloaddition, Takasago
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務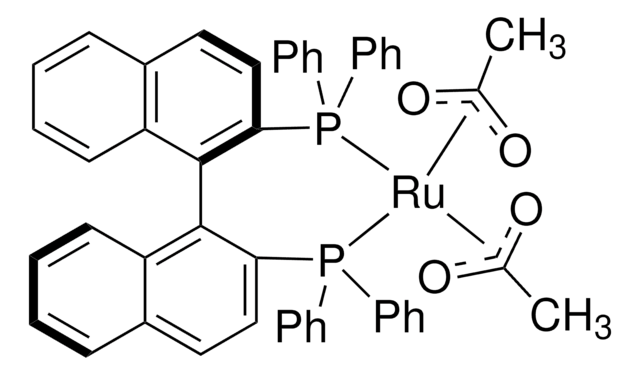


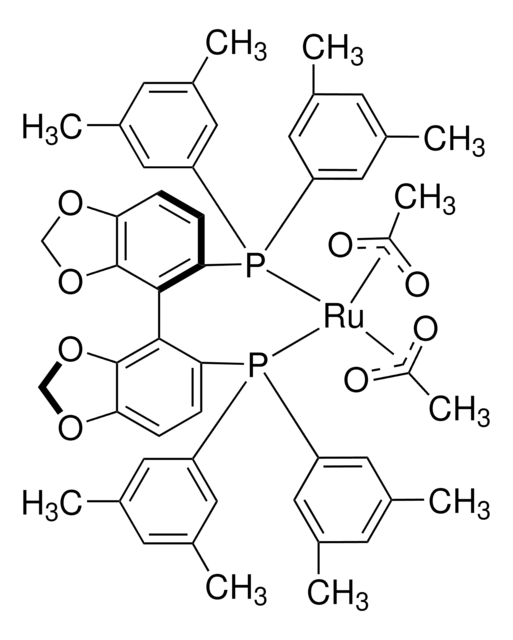


![Ru(OAc)2[(R)-dtbm-SEGPHOS®] Takasago](/deepweb/assets/sigmaaldrich/product/structures/828/527/01325f8f-b95d-42c5-8cca-81e34c63245e/640/01325f8f-b95d-42c5-8cca-81e34c63245e.png)
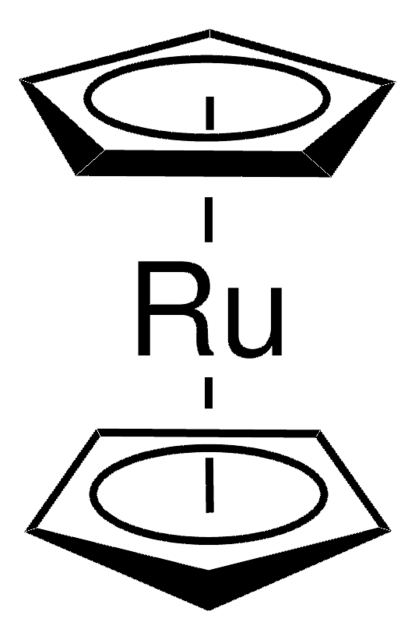
![(R)-RuCl[(p-cymene)(BINAP)]Cl](/deepweb/assets/sigmaaldrich/product/structures/244/078/7a0bdab6-11cc-4030-bbe9-4f687a6a925a/640/7a0bdab6-11cc-4030-bbe9-4f687a6a925a.png)