推薦產品
品質等級
化驗
≥99.0% (calculated, GC, KF)
形狀
liquid
品質
Arxada quality
製造商/商標名
Arxada AG
雜質
≤0.10% water
≤0.50% (E)-2-butenedinitrile
≤0.50% (Z)-2-butenedinitrile
≤0.50% butanedinitrile
bp
220 °C (lit.)
mp
30-32 °C (lit.)
密度
1.049 g/mL at 25 °C (lit.)
官能基
nitrile
儲存溫度
2-8°C
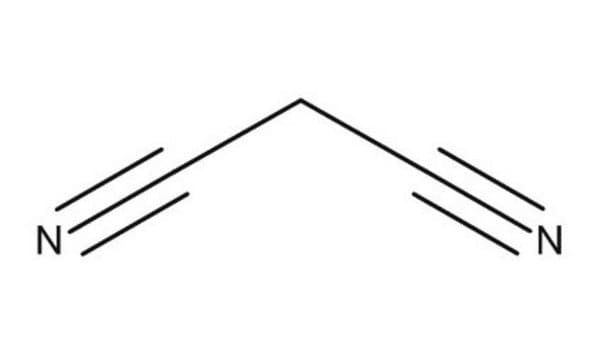
SMILES 字串
N#CCC#N
InChI
1S/C3H2N2/c4-2-1-3-5/h1H2
InChI 密鑰
CUONGYYJJVDODC-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
Some of the reactions where malononitrile is used as a reactant are:
- Synthesis of 2-pyran-4-ylidene-malononitrile (PM) based red light emitting polymers.[5]
- Synthesis of polysubstituted dihydropyridines.[6]
- Synthesis of various chromene derivatives upon treating with salicylic aldehydes.[7]
- Synthesis of triselenium dicyanide by treating it with selenium dioxide.[8]
訊號詞
Danger
危險分類
Acute Tox. 2 Oral - Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Sens. 1
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
186.8 °F - closed cup
閃點(°C)
86 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務



![2- [双(甲硫基)亚甲基]丙二腈 97%](/deepweb/assets/sigmaaldrich/product/structures/144/342/6a420594-3bce-4984-a8b7-5bf2a92d6a97/640/6a420594-3bce-4984-a8b7-5bf2a92d6a97.png)