推薦產品
化驗
97%
形狀
solid
mp
93-100 °C (lit.)
官能基
phenyl
SMILES 字串
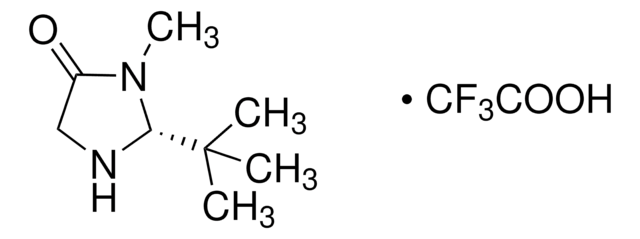
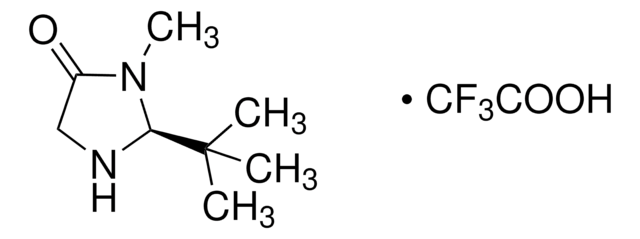
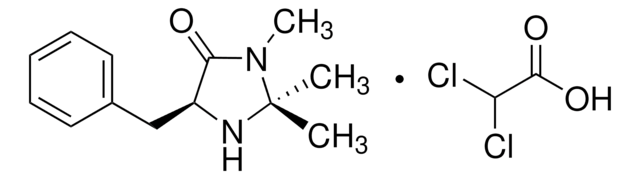
CN1[C@H](N[C@@H](Cc2ccccc2)C1=O)C(C)(C)C
InChI
1S/C15H22N2O/c1-15(2,3)14-16-12(13(18)17(14)4)10-11-8-6-5-7-9-11/h5-9,12,14,16H,10H2,1-4H3/t12-,14-/m0/s1
InChI 密鑰
SKHPYKHVYFTIOI-JSGCOSHPSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
(2S,5S)-(-)-2-叔丁基-3-甲基-5-苄基-4-咪唑啉酮是一种手性咪唑啉酮有机催化剂,由MacMillan及其同事开发。[1]
應用
(2S,5S)-(-)-2-叔丁基-3-甲基-5-苄基-4-咪唑啉酮是第二代麦克米伦催化剂,可作为手性有机催化剂用于:
- 手性转化反应,包括Friedel-Crafts反应和Mukaiyama-Michael反应。[2]
- 通过不对称多米诺Knoevenagel/Diels-Alder反应制备取代螺环烯三酮。[3]
- 通过醛-醛醇醛缩合反应不对称合成β-羟基醛及其二甲基缩醛。[4]
- 使用N-氟苯磺酰胺作为氟化剂,对醛进行对映选择性α-氟化。[5]
- 通过(三烷基甲硅烷氧基)戊二烯醛与呋喃的[4+3]环加成,立体选择性地制备(氧代甲基)氧杂双环[3.2.1]辛烯酮和三环吡咯。[6]
用不对称催化的无金属有机催化剂技术。催化不对称吲哚烷基化、Friedel-Crafts烷基化和高对映体过量的多种共轭加成反应。[7]
特點和優勢
法律資訊
适用美国专利 6,369,243 和相关专利。仅供研究使用。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Teresa D Beeson et al.
Science (New York, N.Y.), 316(5824), 582-585 (2007-03-31)
The asymmetric alpha-addition of relatively nonpolar hydrocarbon substrates, such as allyl and aryl groups, to aldehydes and ketones remains a largely unsolved problem in organic synthesis, despite the wide potential utility of direct routes to such products. We reasoned that
The Importance of Iminium Geometry Control in Enamine Catalysis: Identification of a New Catalyst Architecture for Aldehyde-Aldehyde Couplings
Mangion IK, et al.
Angewandte Chemie (International Edition in English), 116(48), 6890-6892 (2004)
Direct asymmetric α-fluorination of aldehydes
Steiner DD, et al.
Angewandte Chemie (International Edition in English), 117(24), 3772-3776 (2005)
Evaluation of protonation sites in two MacMillan catalysts in solution by gas phase predissociation spectroscopy and electronic structure calculations
Tavares LC, et al.
Organic Chemistry, 134-145 (2020)
Asymmetric organocatalysis of 4+ 3 cycloaddition reactions
Harmata M, et al.
Journal of the American Chemical Society, 125(8), 2058-2059 (2003)
文章
Discover Professor David MacMillan's groundbreaking metal-free asymmetric catalysis using imidazolidinone-based organocatalysts for versatile transformations.
相關內容
In collaboration with Materia, Inc., we are pleased to offer six imidazolidinone OrganoCatalysts™.
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務
![(S)-α,α-双 [3,5-双(三氟甲基)苯基]-2-吡咯烷甲醇三甲基硅醚 97%](/deepweb/assets/sigmaaldrich/product/structures/396/398/09a397b1-b5f5-420f-98da-adf9017cef56/640/09a397b1-b5f5-420f-98da-adf9017cef56.png)


