全部照片(3)
About This Item
經驗公式(希爾表示法):
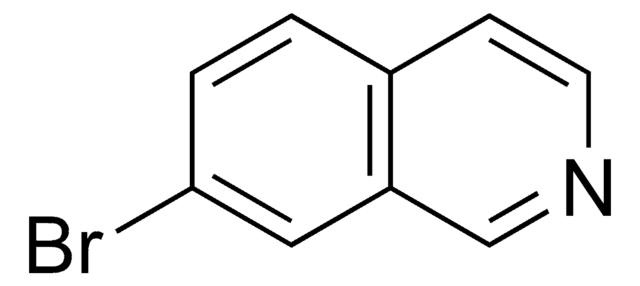
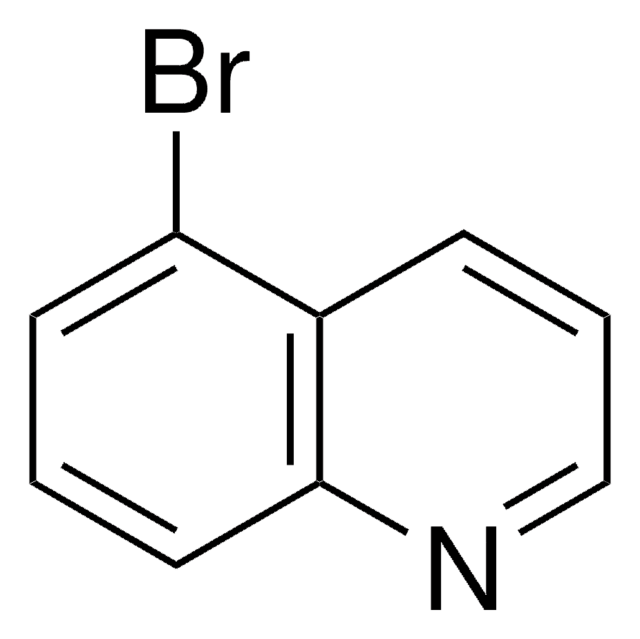
C9H6BrN
CAS號碼:
分子量::
208.05
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推薦產品
化驗
98%
mp
83-87 °C (lit.)
SMILES 字串
Brc1cccc2cnccc12
InChI
1S/C9H6BrN/c10-9-3-1-2-7-6-11-5-4-8(7)9/h1-6H
InChI 密鑰
CYJZJGYYTFQQBY-UHFFFAOYSA-N
應用
用于钯催化的氨甲基化和胺化反应的原料。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Gary A Molander et al.
Organic letters, 9(8), 1597-1600 (2007-03-21)
[reaction: see text] The Suzuki-Miyaura cross-coupling reaction of N,N-dialkylaminomethyltrifluoroborates with aryl halides allows the construction of an aminomethyl aryl linkage through a disconnection based on dissonant reactivity patterns. A variety of these aminomethyltrifluoroborate substrates were prepared in good to excellent
Qilong Shen et al.
Journal of the American Chemical Society, 128(31), 10028-10029 (2006-08-03)
A mild, palladium-catalyzed coupling of aryl halides with ammonia or lithium amide to form primary arylamines as the major product is described. These reactions occurred with excellent selectivity for formation of the primary arylamine over formation of the diarylamine (9.5:1
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務