推薦產品
化驗
97%
折射率
n20/D 1.583 (lit.)
bp
185-188 °C/15 mmHg (lit.)
密度
1.081 g/mL at 25 °C (lit.)
官能基
ketone
phenyl
SMILES 字串
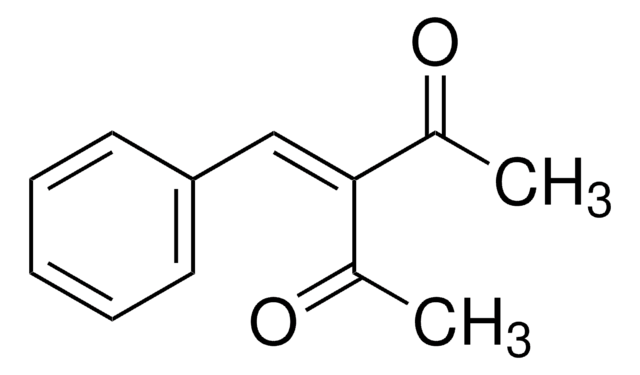
CC(=O)\C(=C\c1ccccc1)C(C)=O
InChI
1S/C12H12O2/c1-9(13)12(10(2)14)8-11-6-4-3-5-7-11/h3-8H,1-2H3
InChI 密鑰
NYRGMNMVISROGJ-UHFFFAOYSA-N
一般說明
3-Benzylidene-2,4-pentanedione (BPD) can be prepared by reacting benzaldehyde with 2,4-pentanedione in the presence of a base.[1]
應用
3-Benzylidene-2,4-pentanedione may be used in the preparation of:
- Five membered cyclic oxyphosphoranes by reacting with phosphonite esters.[2]
- 5-Hydroxy-N-substituted-2H-pyrrol-2-ones by reacting with alkyl isocyanides.[3]
- 3-Benzylidene-2,4-bis (trimethylsilyloxy)-1,4-pentadiene via trimethylsilylation.[1]
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
>230.0 °F - closed cup
閃點(°C)
> 110 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
5-Hydroxy-2H-pyrrol-2-ones and not 2-aminofurans are the cycloaddition products between alkyl isocyanides and benzyliden-1,3-diketones.
Quai M, et al.
Tetrahedron Letters, 45(7), 1413-1416 (2004)
3-Benzylidene-2, 4-bis (trimethylsilyloxy)-1, 4-pentadiene; Synthesis and its diene-transmissive Diels-Alder reaction.
Tsuge O, et al.
Chemistry Letters (Jpn), 2, 239-242 (1983)
Stereoisomerism at phosphorus in cyclic oxyphosphoranes. Reaction of phosphonite and phosphinite esters with 3-benzylidene-2, 4-pentanedione.
Ramirez F, et al.
Journal of the American Chemical Society, 90(5), 1275-1280 (1968)
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務