推薦產品
化驗
98%
mp
73-76 °C (lit.)
官能基
amine
chloro
oxime
SMILES 字串
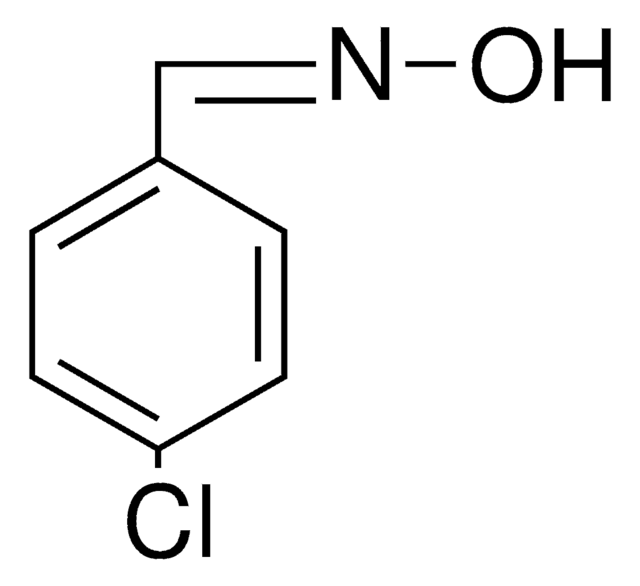
O\N=C\c1ccccc1Cl
InChI
1S/C7H6ClNO/c8-7-4-2-1-3-6(7)5-9-10/h1-5,10H/b9-5+
InChI 密鑰
FZIVKDWRLLMSEJ-WEVVVXLNSA-N
一般說明
2-Chlorobenzaldehyde oxime is also known as o-chlorobenzaldehyde oxime. It can be synthesized by reacting 2-chlorobenzaldehyde and hydroxylamine hydrochloride.[1]
應用
2-Chlorobenzaldehyde oxime may be used in the preparation of:
- 2-chlorobenzaldehyde under different reaction conditions[2][3][4][5]
- methyl 3-(2-chlorophenyl)-5-[1-(4-methoxybenzyloxy)-ethyl]isoxazole-4-carboxylate[6]
- dimethyl 3-(2-chlorophenyl)isoxazole-4,5-dicarboxylate[1]
- [3-(2-chlorophenyl)isoxazol-5-yl]methanol[7]
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Amberlyst 15 supported nitrosonium ion as an efficient reagent for regeneration of carbonyl compounds from oximes, hydrazones and semicarbazones.
Lakouraj MM, et al.
Reactive functional Polymers, 66(9), 910-915 (2006)
Microwave-assisted chemoselective cleavage of oximes to their corresponding carbonyl compounds using 1, 3-dichloro-5, 5-dimethyl-hydantoin (DCDMH) as a new Deoximating reagent.
Khazaei A and Manesh AA.
Synthesis, 2005(12), 1929-1931 (2005)
Hypervalent iodine mediated synthesis of di-and tri-substituted isoxazoles via [3+2] cycloaddition of nitrile oxides.
Singhal A, et al.
Tetrahedron, 57(7), 719-722 (2016)
Design, synthesis and antibacterial activity of novel N-formylhydroxylamine derivatives as PDF inhibitors.
Yin L, et al.
Indian J. Chem. B, 50(5), 695-695 (2011)
Facile and Chemoselective Microwave-Assisted Cleavage of Oximes to Their Corresponding Carbonyl Compounds Using N,N?-Dibromo-N,N?-1,3-propylene-bis[(4-methylphenyl)sulfonamide] as a Deoximating Reagent.
Khazaei A, et al.
Synthesis, 2004(17), 2784-2786 (2004)
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務