推薦產品
化驗
97%
折射率
n20/D 1.48 (lit.)
bp
130-133 °C/4 mmHg (lit.)
mp
17 °C (lit.)
密度
1.081 g/mL at 25 °C (lit.)
SMILES 字串
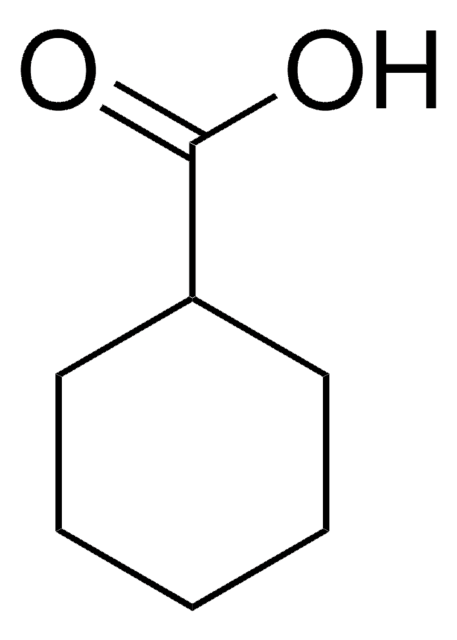
OC(=O)C1CCC=CC1
InChI
1S/C7H10O2/c8-7(9)6-4-2-1-3-5-6/h1-2,6H,3-5H2,(H,8,9)
InChI 密鑰
VUSWCWPCANWBFG-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Dermal - Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
230.0 °F - closed cup
閃點(°C)
110 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Yi Yang et al.
Environmental science & technology, 48(4), 2344-2351 (2014-02-01)
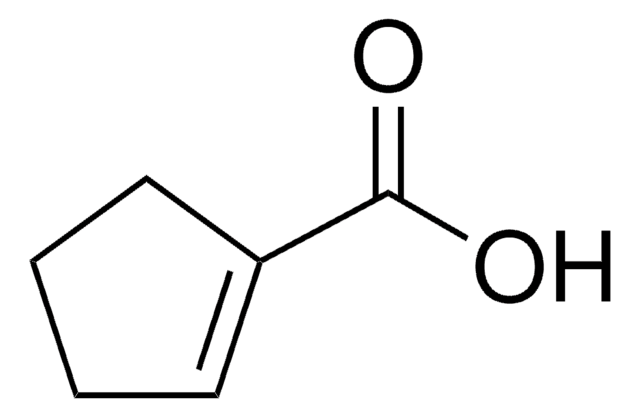
The effect of halides on organic contaminant destruction efficiency was compared for UV/H2O2 and UV/S2O8(2-) AOP treatments of saline waters; benzoic acid, 3-cyclohexene-1-carboxylic acid, and cyclohexanecarboxylic acid were used as models for aromatic, alkene, and alkane constituents of naphthenic acids
Bromination of 3-cyclohexene-1-carboxylic acid, epoxydation of methyl 3-cyclohexene-1-carboxylate and opening of methyl cis-and trans-3, 4-epoxycyclohexane-1-carboxylate: Stereochemical results.
Bellucci G, et al.
Tetrahedron, 28(13), 3393-3399 (1972)
Total synthesis of (.+-.)-methyl shikimate and (.+-.)-3-phosphoshikimic acid.
Bartlett PA and McQuaid LA.
Journal of the American Chemical Society, 106(25), 7854-7860 (1984)
Yeon Hee Ban et al.
Molecular bioSystems, 9(5), 944-947 (2012-12-12)
A FK506 analogue containing a non-natural starter unit was obtained through mutasynthesis by feeding cultures of Streptomyces sp. KCTC 11604BP fkbO deletion mutant with 3-cyclohexene-1-carboxylic acid. The structure of the new compound, 32-dehydroxy-FK506, and its biological activities were determined.
Karine Barral et al.
Journal of medicinal chemistry, 48(2), 450-456 (2005-01-22)
Starting from commercially available (rac)-3-cyclohexene-1-carboxylic acid, a series of purine and pyrimidine cis-substituted cyclohexenyl and cyclohexanyl nucleosides were synthesized through a key Mitsunobu reaction. Antiviral evaluations were performed on HIV, coxsackie B3, and herpes viruses (HSV-1, HSV-2, VZV, HCMV). Three
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務