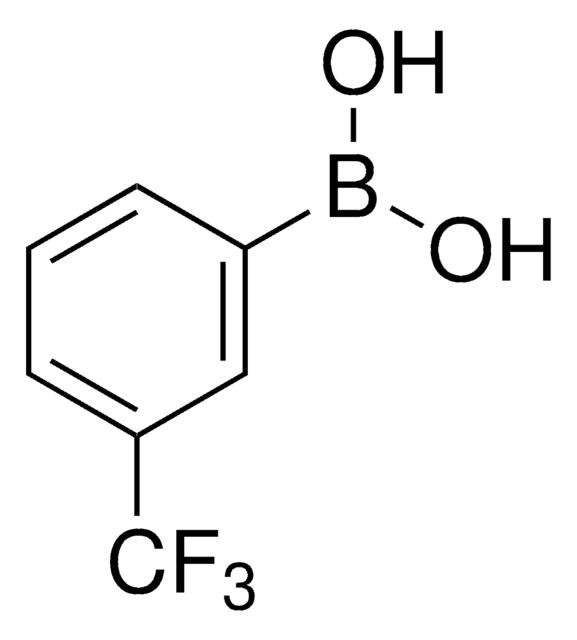
推薦產品
化驗
≥95.0%
形狀
solid
mp
111-114 °C (lit.)
官能基
fluoro
SMILES 字串
OB(O)c1ccccc1C(F)(F)F
InChI
1S/C7H6BF3O2/c9-7(10,11)5-3-1-2-4-6(5)8(12)13/h1-4,12-13H
InChI 密鑰
JNSBEPKGFVENFS-UHFFFAOYSA-N
應用
2-(Trifluoromethyl)phenylboronic acid can be used as a reactant:
- In Suzuki-coupling reactions to prepare 2-trifluoromethyl aryl or heteroaryl derivatives.[1]
- To synthesize 4-(2-trifluoromethyl)phenylpyrrolo[2,3-d]pyrimidine as a potential antagonist of corticotropin-releasing hormone.[2]
- To prepare 2-nitro-6-(trifluoromethyl)phenylboronic acid by nitration reaction.[3]
其他說明
含不定量的酸酐
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
客戶也查看了
Studies of palladium-catalyzed coupling reactions for preparation of hindered 3-arylpyrroles relevant to (-)-rhazinilam and its analogues
Ghosez L, et al.
Canadian Journal of Chemistry, 79(11), 1827-1839 (2001)
Efficient synthetic approach to heterocycles possessing the 3, 3-disubstituted-2, 3-dihydrobenzofuran skeleton via diverse palladium-catalyzed tandem reactions
Szlosek-Pinaud M, et al.
Tetrahedron, 63(16), 3340-3349 (2007)
Functionalization of pyrrolo [2, 3-d] pyrimidine by palladium-catalyzed cross-coupling reactions
Tumkevicius, S and Dodonova, J
Chemistry of Heterocyclic Compounds, 48(2), 258-279 (2012)
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務