推薦產品
化驗
≥99%
折射率
n20/D 1.582 (lit.)
bp
89 °C/16 mmHg (lit.)
mp
−8 °C (lit.)
密度
0.997 g/mL at 25 °C (lit.)
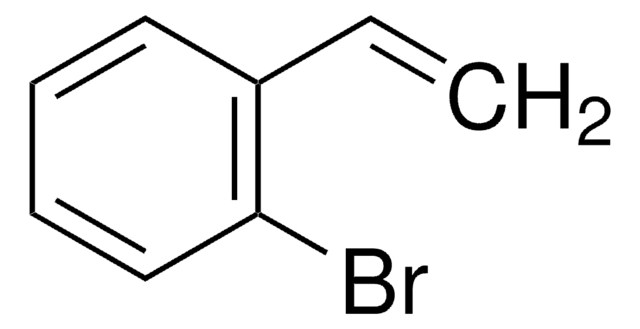
SMILES 字串
C1Cc2ccccc2C=C1
InChI
1S/C10H10/c1-2-6-10-8-4-3-7-9(10)5-1/h1-3,5-7H,4,8H2
InChI 密鑰
KEIFWROAQVVDBN-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
取代透過
產品號碼
描述
訂價
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
152.6 °F - closed cup
閃點(°C)
67 °C - closed cup
個人防護裝備
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
D S Torok et al.
Journal of bacteriology, 177(20), 5799-5805 (1995-10-01)
Bacterial strains expressing toluene and naphthalene dioxygenase were used to examine the sequence of reactions involved in the oxidation of 1,2-dihydronaphthalene. Toluene dioxygenase of Pseudomonas putida F39/D oxidizes 1,2-dihydronaphthalene to (+)-cis-(1S,2R)-dihydroxy-1,2,3,4-tetrahydronaphthalene, (+)-(1R)-hydroxy-1,2-dihydronaphthalene, and (+)-cis-(1R,2S)-dihydroxy-1,2-dihydronaphthalene. In contrast, naphthalene dioxygenase of Pseudomonas
S L Eaton et al.
Applied and environmental microbiology, 62(12), 4388-4394 (1996-12-01)
The substrate oxidation profiles of Sphingomonas yanoikuyae B1 biphenyl-2,3-dioxygenase and cis-biphenyl dihydrodiol dehydrogenase activities were examined with 1,2-dihydronaphthalene and various cis-diols as substrates. m-Xylene-induced cells of strain B1 oxidized 1,2-dihydronaphthalene to (-)-(1R,2S)-cis-1,2-dihydroxy-1,2-3,4-tetrahydronaphthalene as the major product (73% relative yield). Small
Keith Smith et al.
Chemical communications (Cambridge, England), (8)(8), 886-887 (2002-07-19)
We have successfully prepared an unsymmetrical analogue of a Katsuki-type salen ligand having a single hydroxyalkyl group at its 6-position, and also its Mn(III) complex; attachment of the complex to a polymer gives a highly enantioselective and recoverable catalyst for
Claudia Sanfilippo et al.
Biotechnology letters, 26(23), 1815-1819 (2005-01-27)
Chloroperoxidase from Caldariomyces fumago catalyses the oxidation of 1,2-dihydronaphthalene to (1R,2R)-(+)-dihydroxytetrahydronaphthalene in homogenous citrate buffer/ionic liquid mixtures, using t-butyl hydroperoxide as O2 donor. It tolerates up to 30 (v/v) 1,3-dimethylimidazolium methylsulfate or 1-butyl-3-methylimidazolium methylsulfate. The enzyme activity in these ionic
Márcia Kameyama et al.
Molecules (Basel, Switzerland), 16(11), 9421-9438 (2011-11-15)
A new approach for the synthesis of indatraline was developed using as the key step an iodine(III)-mediated ring contraction of a 1,2-dihydronaphthalene derivative. Behavioral tests were conducted to evaluate the effect of indatraline and of its precursor indanamide on the
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務