全部照片(1)
About This Item
線性公式:
(C6H11)2BI
CAS號碼:
分子量::
304.02
MDL號碼:
分類程式碼代碼:
12352101
PubChem物質ID:
NACRES:
NA.22
暫時無法取得訂價和供貨情況
推薦產品
化驗
97%
形狀
liquid
反應適用性
reagent type: reductant
bp
198-200 °C/1.25 mmHg (lit.)
密度
1.325 g/mL at 25 °C (lit.)
SMILES 字串
IB(C1CCCCC1)C2CCCCC2
InChI
1S/C12H22BI/c14-13(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h11-12H,1-10H2
InChI 密鑰
RWFGGTOYIFQXAO-UHFFFAOYSA-N
應用
Dicyclohexyliodoborane (Chx2BI) can be used as a reagent:
- For the enolboration of esters and tertiary amides to synthesize corresponding Z or E enolates.[1][2]
- In the stereoselective preparation of β-hydroxy-α-trifluoromethyl carboxylic acids via haloborane-mediated diastereoselective aldol addition of aldehydes with trifluoropropanoic acid.[3]
- In the total synthesis of pordamacrine A,[4] and trocheliophorolide D.[5]
Reactant for:
- Preparation of vinyloxyboranes
Enolboration. 6. Dicyclohexyliodoborane, a Versatile Reagent for the Stereoselective Synthesis of Either Z or E Enolates from Representative Esters
Ganesan K and Brown HC
The Journal of Organic Chemistry, 59(9), 2336-2340 (1994)
Dicyclohexyliodoborane/Triethylamine-a new reagent which achieves the facile enolboration of esters and tertiary amides
Brown HC and Ganesan K
Tetrahedron Letters, 33(24), 3421-3424 (1992)
Total Synthesis of the Proposed Structure of Trocheliophorolide D
Hwang S, et al.
European Journal of Organic Chemistry, 2011(36), 7414-7418 (2011)
A boron-based Ireland-Claisen approach to the synthesis of pordamacrine A
Seizert, Curtis A and Ferreira, Eric M
Tetrahedron, 73(29), 4186-4194 (2017)
Diastereoselective synthesis of anti-3-hydroxy-2-trifluoromethyl carboxylic acids
Ramachandran PV, et al.
Tetrahedron Letters, 56(23), 3019-3022 (2015)
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務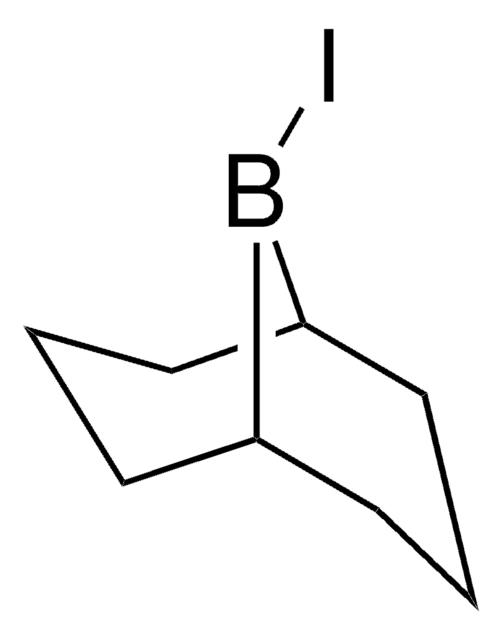
![9-硼双环[3.3.1]壬烷 溶液 0.5 M in THF](/deepweb/assets/sigmaaldrich/product/structures/180/891/8b64e597-269d-4780-98b6-40889dfd06b9/640/8b64e597-269d-4780-98b6-40889dfd06b9.png)




![9-硼双环[3.3.1]壬烷二聚体](/deepweb/assets/sigmaaldrich/product/structures/203/431/624973a6-aec1-4b23-b6c4-013285ac418c/640/624973a6-aec1-4b23-b6c4-013285ac418c.png)