全部照片(1)
About This Item
經驗公式(希爾表示法):
C10H10O
CAS號碼:
分子量::
146.19
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
暫時無法取得訂價和供貨情況
推薦產品
化驗
99%
形狀
liquid
折射率
n20/D 1.555 (lit.)
bp
93-95 °C/4 mmHg (lit.)
密度
1.064 g/mL at 25 °C (lit.)
官能基
ketone
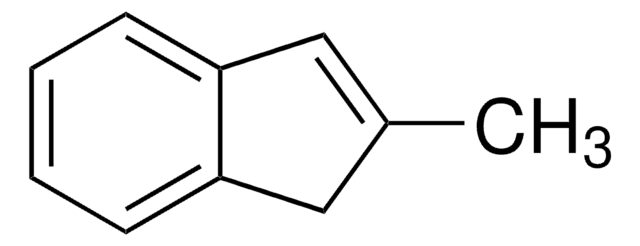
SMILES 字串
CC1Cc2ccccc2C1=O
InChI
1S/C10H10O/c1-7-6-8-4-2-3-5-9(8)10(7)11/h2-5,7H,6H2,1H3
InChI 密鑰
BEKNOGMQVKBMQN-UHFFFAOYSA-N
一般說明
2-Methyl-1-indanone, a α-benzocycloalkenone[1], is a derivative of 1-indanone. Its synthesis has been reported.[2][3][4] The enzymatic dynamic kinetic resolution (DKR) of racemic 2-methyl-1-indanone has been studied.[5] The asymmetric α-arylation[6] and hydroxymethylation of 2-methyl-1-indanone has been reported.[7] It participated in the synthesis of 2-methyl-6-carboxyazulene.[8]
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves
On the decarboxylation of 2-methyl-1-tetralone-2-carboxylic acid-oxidation of the enol intermediate by triplet oxygen.
Riahi A, et al.
New. J. Chem., 37(8), 2245-2249 (2013)
Shaozhong Ge et al.
Journal of the American Chemical Society, 133(41), 16330-16333 (2011-09-16)
We report the α-arylation of ketones with a range of aryl chlorides with enantioselectivities from 90 to 99% ee catalyzed by the combination of Ni(COD)(2) and (R)-BINAP and the coupling of ketones with a range of heteroaryl chlorides with enantioselectivities
Michael W Justik et al.
Molecules (Basel, Switzerland), 10(1), 217-225 (2007-11-17)
The conversion of alpha-benzocycloalkenones to homologous beta-benzocyclo-alkenones containing six, seven and eight-membered rings is reported. This was accomplished via a Wittig olefination-oxidative rearrangement sequence using[hydroxy(tosyloxy)iodo]-benzene (HTIB) is the oxidant, that enables the synthesis of regioisomeric pairs of methyl-substituted beta-benzocycloalkenones. The
Michał Rachwalski et al.
Chemical Society reviews, 42(24), 9268-9282 (2013-09-26)
Deracemisation of racemic compounds is still the most important strategy to produce optically pure compounds despite many recent advances in asymmetric synthesis. Especially deracemisation approaches that give rise to single enantiomers are preferred, which can be achieved either by invoking
Taku Kitanosono et al.
Chemistry, an Asian journal, 10(1), 133-138 (2014-10-29)
Enzymes exhibit overwhelmingly superior catalysis compared with artificial catalysts. Current strategies to rival enzymatic catalysis require unmodified or minimally modified structures of active sites, gigantic molecular weight, and sometimes the use of harsh conditions such as extremely low temperatures in
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務