全部照片(1)
About This Item
經驗公式(希爾表示法):
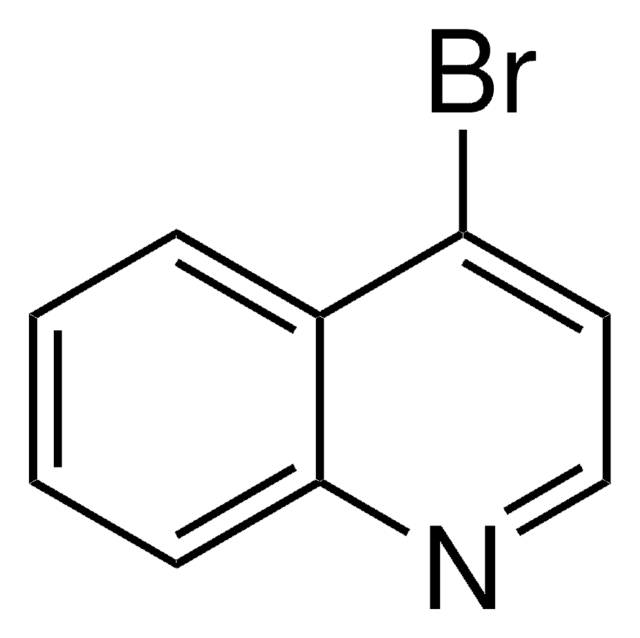
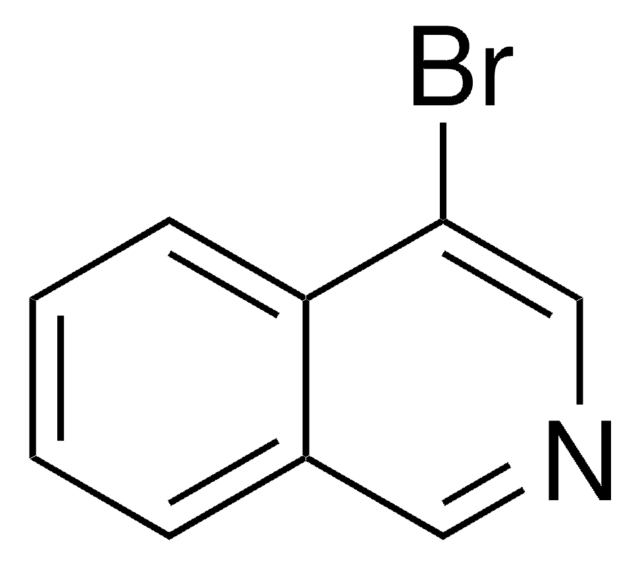
C9H6BrN
CAS號碼:
分子量::
208.05
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
暫時無法取得訂價和供貨情況
推薦產品
化驗
98%
折射率
n20/D 1.672 (lit.)
bp
112-113 °C/0.5 mmHg (lit.)
密度
1.594 g/mL at 25 °C (lit.)
官能基
bromo
SMILES 字串
Brc1cccc2cccnc12
InChI
1S/C9H6BrN/c10-8-5-1-3-7-4-2-6-11-9(7)8/h1-6H
InChI 密鑰
PIWNKSHCLTZKSZ-UHFFFAOYSA-N
一般說明
8-溴喹啉是喹诺酮衍生物。它被广泛用于染料、食用色素、药物试剂、pH 指示剂的合成以及各种工业过程。其分子带有吡啶基。在钯催化剂的存在下,它与各种杂芳族化合物发生直接杂芳基化反应,得到聚杂芳族衍生物。[1]
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Jung-Ho Son et al.
Dalton transactions (Cambridge, England : 2003), 39(45), 11081-11090 (2010-10-23)
The ambiphilic molecule 8-(dimesitylboryl)quinoline (1) was synthesized by treatment of 8-bromoquinoline or 8-iodoquinoline with n-BuLi followed by dimesitylboronfluoride. Hydrolysis of 1 is unusually rapid compared to bulky triorganoboranes with the sequential loss of mesitylene and formation of mesityl(quinolin-8-yl)borinic acid (2)
Facile synthesis of 8-substituted quinolines.
Suggs JW and Pearson GDN.
The Journal of Organic Chemistry, 45(8), 1514-1515 (1980)
Direct heteroarylation of 5-bromothiophen-2-ylpyridine and of 8-bromoquinoline via palladium-catalysed C-H bond activation: simpler access to heteroarylated nitrogen-based derivatives.
Laroche J, et al.
Catalysis Science & Technology, 3(8), 2072-2080 (2013)
Sébastien Noël et al.
Organic & biomolecular chemistry, 4(20), 3760-3762 (2006-10-07)
Unexpectedly, the palladium catalyzed coupling reaction of acrolein with 8-bromoquinoline gave 5H-pyrido[3,2,1-ij]quinolin-3-one in a single step.
Synthesis of n, n'-biquinolines by a coupling reaction of bromoquinolines using organonickel (0) complexes.
Benito Y, et al.
Applied Organometallic Chemistry, 1(6), 535-540 (1987)
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務