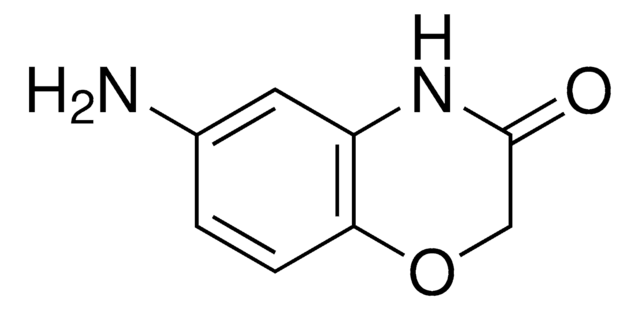
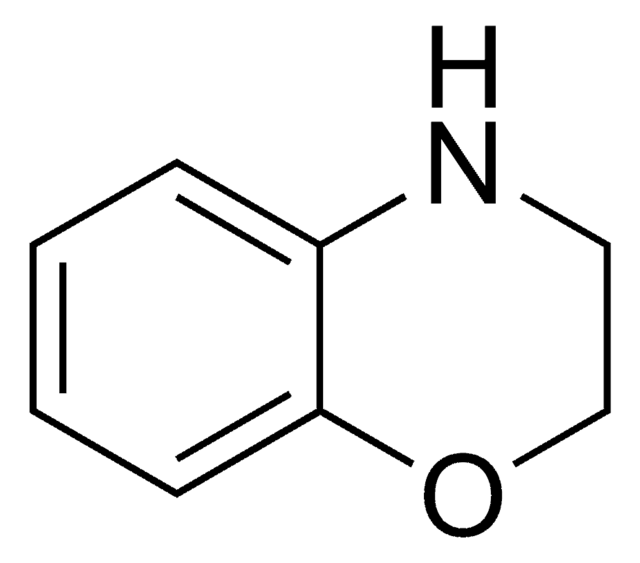
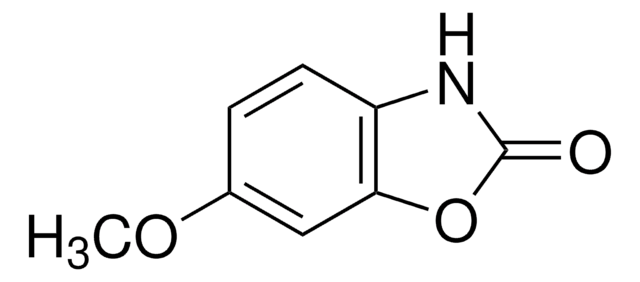
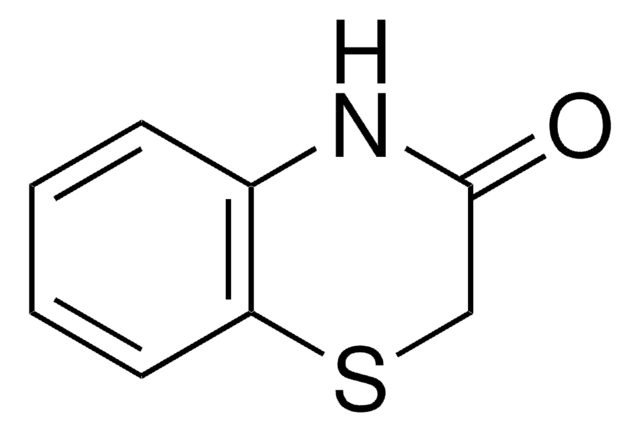
推薦產品
化驗
99%
mp
173-175 °C (lit.)
溶解度
methanol: soluble 25 mg/mL, clear, colorless
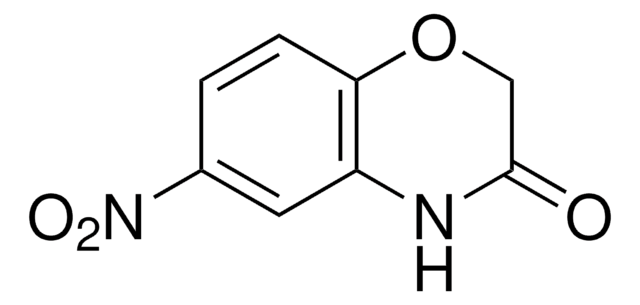
SMILES 字串
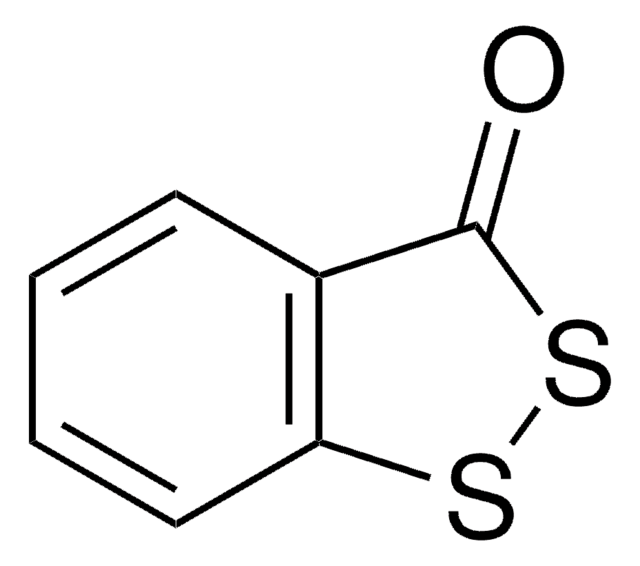
O=C1COc2ccccc2N1
InChI
1S/C8H7NO2/c10-8-5-11-7-4-2-1-3-6(7)9-8/h1-4H,5H2,(H,9,10)
InChI 密鑰
QRCGFTXRXYMJOS-UHFFFAOYSA-N
1 of 4
本產品 | 375462 | 561487 | 746037 |
|---|---|---|---|
| Quality Level 100 | Quality Level 100 | Quality Level 100 | Quality Level 100 |
| solubility methanol: soluble 25 mg/mL, clear, colorless | solubility toluene: soluble 2.5%, clear, yellow | solubility - | solubility - |
| mp 173-175 °C (lit.) | mp 74-77 °C (lit.) | mp 154-158 °C (lit.) | mp 88-93 °C |
一般說明
2H-1,4-Benzoxazin-3(4H)-one, a benzoxazine derivative, is a heterocyclic building block for various natural and synthetic organic compounds.[1] It has been reported as an intermediate during the biogenesis of cyclic hydoxamic acids in maize.[2] Its standard molar enthalpy of formation and tautomerization energy of its tautomers has been evaluated by calorimetric and computational methods.[3] It has been synthesized by reacting o-aminophenol with chloroacetyl chloride in the presence of butanone and aqueous NaHCO3.[4]
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Calorimetric and computational study of 2H-1,4-benzoxazin-3(4H)-one and of related species.
Matos MAR, et al.
Molecular Physics, 104(12), 1833-1841 (2006)
Jiu Hong Wu et al.
Bioorganic & medicinal chemistry letters, 13(13), 2223-2225 (2003-06-12)
A new inhibitor of in vitro tumor cell replication, cappamensin A (1) (2H-1,4-benzoxazin-3(4H)-one, 6-methoxy-2-methyl-4-carbaldehyde), was isolated from the roots of Capparis sikkimensis subsp. formosana using bioactivity-guided fractionation. The structure of 1 was established by spectroscopic methods, including 2D NMR analyses.
2H-1,4-benzoxazin-3(4H)-one, an intermediate in the biosynthesis of cyclic hydroxamic acids in maize.
Kumar P, et al.
Phytochemistry, 36(4), 893-898 (1994)
A general and convenient synthesis of 2H-1,4-benzoxazin-3(4H)-ones.
Shridhar DR, et al.
Organic Prep. and Proc. Int., 14(3), 195-197 (1982)
Moreshwar B Chaudhari et al.
The Journal of organic chemistry, 85(5), 3374-3382 (2020-01-31)
We report here the Sn-catalyzed mild protocol for ring expansion of peroxyoxindoles to afford the series of substituted-2H-benzo[b][1,4]oxazin-3(4H)-one derivatives. In this protocol, we showed the in situ conversion of tert-butyl peroxy compounds into peresters with the aid of external esters
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務