推薦產品
產品名稱
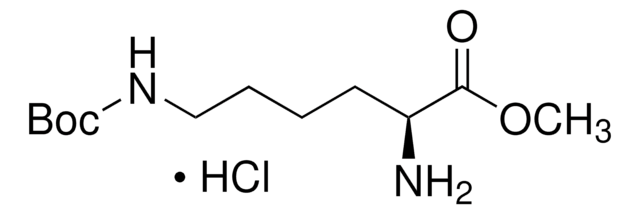
H-Lys(Boc)-OH, ≥95%
品質等級
化驗
≥95%
形狀
powder
光學活性
[α]20/D +18°, c = 1 in acetic acid
反應適用性
reaction type: solution phase peptide synthesis
mp
250 °C (dec.) (lit.)
應用
peptide synthesis
儲存溫度
2-8°C
SMILES 字串
CC(C)(C)OC(=O)NCCCC[C@H](N)C(O)=O
InChI
1S/C11H22N2O4/c1-11(2,3)17-10(16)13-7-5-4-6-8(12)9(14)15/h8H,4-7,12H2,1-3H3,(H,13,16)(H,14,15)/t8-/m0/s1
InChI 密鑰
VVQIIIAZJXTLRE-QMMMGPOBSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
H-Lys(Boc)-OH又称为Nε-Boc-L-赖氨酸,常用于液相肽合成。
應用
H-Lys(Boc)-OH可制备五氟苯酯用于进一步合成β-肽。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
從最近期的版本中選擇一個:
分析證明 (COA)
Lot/Batch Number
客戶也查看了
Solution phase synthesis of beta-peptides using micro reactors
P Watts
Tetrahedron, 58, 5427-5439 (2002)
Ognyan K Argirov et al.
Biochimica et biophysica acta, 1620(1-3), 235-244 (2003-02-22)
Proteins are subject of posttranslational modification by sugars and their degradation products in vivo. The process is often referred as glycation. L-Dehydroascorbic acid (DHA), an oxidation product of L-ascorbic acid (vitamin C), is known as a potent glycation agent. A
K Itakura et al.
Chemical research in toxicology, 14(5), 473-475 (2001-05-23)
It has been suggested that protein modifications by malondialdehyde (MDA), a major product of lipid peroxidation, contribute to the fluorescence formation of lipofuscin. Although early studies proposed an aminoenimine structure (RNHCH=CHCH=NR) formed from MDA and the epsilon-amino groups of the
J T Sparrow et al.
Peptide research, 9(6), 297-304 (1996-11-01)
We have synthesized a hydrophilic crosslinked aminoalkyl polydimethylacrylamide-beaded support upon which peptides have been assembled using standard Fmoc chemistry in automated batch-wise equipment. The resin was prepared by the free radical-initiated co-polymerization of N,N-dimethylacryl-amide, N,N'-bisacrylyl-1,3-diaminopropane and a functional monomer N-methacrylyl-1,3-diaminopropane
April Case et al.
Analytical biochemistry, 338(2), 237-244 (2005-03-05)
Tissue transglutaminase (TGase) is a Ca(2+)-dependent enzyme that catalyzes cross-linking of intracellular proteins through a mechanism that involves isopeptide bond formation between Gln and Lys residues. In addition to its transamidation activity, TGase can bind guanosine 5'-triphosphate (GTP) and does
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務