推薦產品
蒸汽密度
3.8 (vs air)
蒸汽壓力
26.9 mmHg ( 37.7 °C)
7.2 mmHg ( 20 °C)
化驗
96%
形狀
liquid
自燃溫度
559 °F
折射率
n20/D 1.476 (lit.)
bp
132-134 °C (lit.)
mp
11-14 °C (lit.)
密度
0.773 g/mL at 25 °C (lit.)
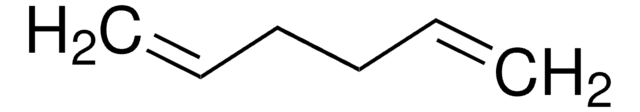
SMILES 字串
C\C(C)=C/C=C(\C)C
InChI
1S/C8H14/c1-7(2)5-6-8(3)4/h5-6H,1-4H3
InChI 密鑰
DZPCYXCBXGQBRN-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
訊號詞
Warning
危險分類
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
84.2 °F - closed cup
閃點(°C)
29 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Katsuhiro Suenobu et al.
Journal of the American Chemical Society, 126(23), 7271-7280 (2004-06-10)
The reaction pathway and the mechanism of asymmetric induction in the synthesis of (+)-trans-(1R,3R)-chrysanthemic acid methyl ester from methyl diazoacetate and 2,5-dimethyl-2,4-hexadiene in the presence of a C(1)-chiral salicylaldimine Cu(I) complex has been probed with the aid of hybrid density
Maki Ohashi et al.
Organic letters, 10(13), 2741-2743 (2008-06-10)
The synthesis of monoalkylated propanedinitriles was achieved upon photoirradiation of MeCN/H(2)O solutions containing propanedinitrile (1; malononitrile) and electron-rich alkenes in the presence of lithium carbonate and a catalytic amount of 9-cyanophenanthrene or redox-type photosensitizers (electron-mediating photosensitizers), through regioselective anti-Markovnikov photochemical
Makoto Itagaki et al.
The Journal of organic chemistry, 70(8), 3292-3295 (2005-04-13)
Some new bisoxazoline ligands with an aryl group at the 4-position and gem-dimethyl groups at the 5-position on the oxazoline ring were prepared from arylglycines. Remarkable enhancement of the trans-selectivity (trans/cis = 87/13) and the enantioselectivity (96% ee for the
J Saltiel et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 8(6), 856-867 (2009-06-06)
Photochemical formation of 9-chloroanthracene (MCA) from 9,10-dichloroanthracene (DCA) is observed in the presence of 2,5-dimethyl-2,4-hexadiene (DMH) in acetonitrile (AN). The mechanism of the reaction was investigated using kinetics, deuterium labeling, and quenching techniques. Contrary to conclusions in a recent publication
Organic sulfur compounds. VII. Some addition and co-oxidation reactions of thiols with 2, 5-dimethyl-2, 4-hexadiene.
Oswald AA, et al.
The Journal of Organic Chemistry, 27(7), 2439-2448 (1962)
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務