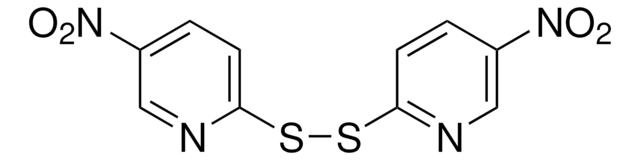
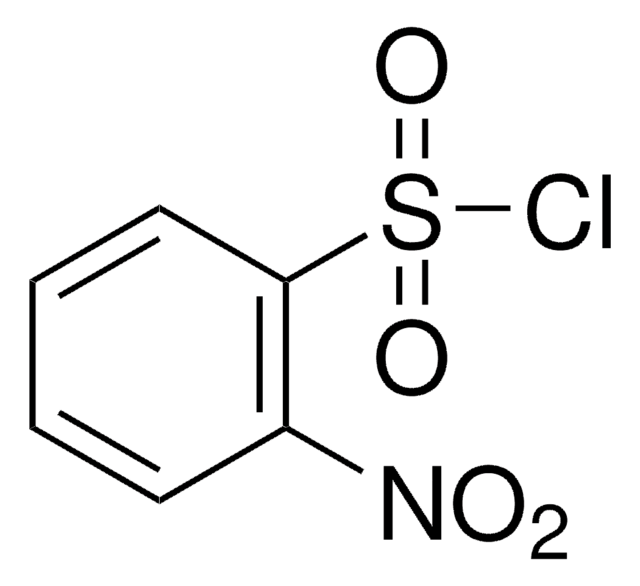
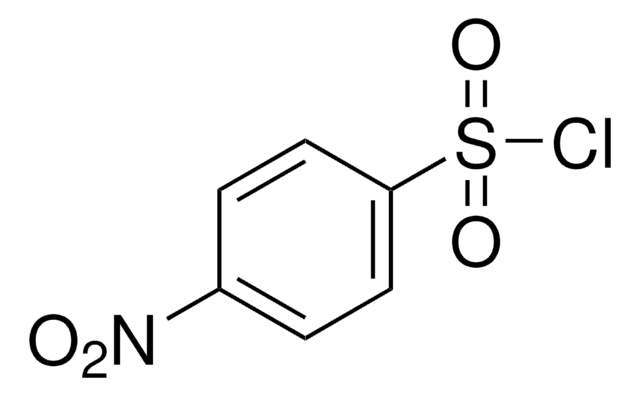
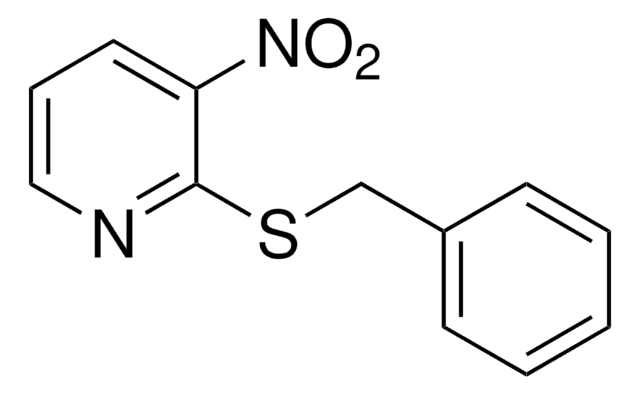
推薦產品
化驗
95%
mp
205 °C (dec.) (lit.)
溶解度
dichloromethane: soluble(lit.)
官能基
nitro
儲存溫度
2-8°C
SMILES 字串
[O-][N+](=O)c1cccnc1SCl
InChI
1S/C5H3ClN2O2S/c6-11-5-4(8(9)10)2-1-3-7-5/h1-3H
InChI 密鑰
WTKQMHWYSBWUBE-UHFFFAOYSA-N
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
R Matsueda et al.
Peptide research, 5(5), 262-264 (1992-09-01)
Two recent reports on the partial lability of the 3-nitro-2-pyridinesulfenyl (Npys) thiol protecting group towards 1-hydroxy-benzotriazole (HOBt) have prompted a rechecking of the chemical behavior of this group. Using both soluble and polymer-bound forms of Cys(Npys) as test materials, the
R G Simmonds et al.
International journal of peptide and protein research, 43(4), 363-366 (1994-04-01)
The 3-nitro-2-pyridinesulphenyl (Npys) moiety is finding increasing utility as a protecting-activating group for cysteine, particularly in the synthesis of cyclic and unsymmetrical disulfides using the Boc strategy. This chemistry has been extended to peptides assembled by the Fmoc strategy. N-Terminal
S Rajagopalan et al.
International journal of peptide and protein research, 45(2), 173-179 (1995-02-01)
TASPs (template-assembled synthetic peptides) are generated by the covalent attachment of linear peptides to a common peptide backbone, thus generating larger synthetic peptides/proteins with prefolded structure. In this work we present a strategy for the synthesis of a heterotemplate-assembled synthetic
K C Pugh et al.
International journal of peptide and protein research, 42(2), 159-164 (1993-08-01)
3-Nitro-2-pyridinesulfenyl chloride (NpysCl) is the starting material for the synthesis of N-, O- and S-Npys-protected amino acids. Two efficient, novel synthetic routes to NpysCl are described. The stability of NpysCl was determined in a variety of solvents, with and without
O Rosen et al.
International journal of peptide and protein research, 35(6), 545-549 (1990-06-01)
The hydroxylic side-chain functional groups of serine, threonine, hydroxproline and tyrosine, the alpha and epsilon-amino moieties of lysine and the thiol group of cysteine were masked by the 3-nitro-2-pyridinesulfenyl (Npys) protecting group. Deprotection was mildly affected by thiolysis with either
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務