全部照片(1)
About This Item
經驗公式(希爾表示法):
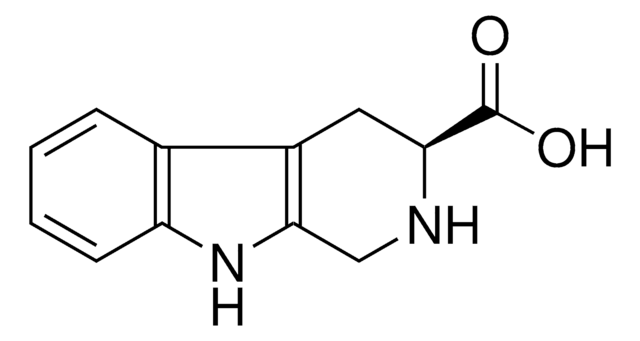
C11H12N2
CAS號碼:
分子量::
172.23
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
暫時無法取得訂價和供貨情況
推薦產品
化驗
98%
形狀
liquid
mp
206-208 °C (lit.)
SMILES 字串
C1Cc2c(CN1)[nH]c3ccccc23
InChI
1S/C11H12N2/c1-2-4-10-8(3-1)9-5-6-12-7-11(9)13-10/h1-4,12-13H,5-7H2
InChI 密鑰
CFTOTSJVQRFXOF-UHFFFAOYSA-N
基因資訊
rat ... Htr2a(29595) , Htr2c(25187)
一般說明
Ozonolysis of the enamine bond of 1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole derivatives was studied.[1]
應用
- Reactant for synthesis of the indolyl-β-carboline alkaloid eudistomin U via IBX mediated room temperature oxidative aromatization
- Reactant for preparation of neuroprotective HDAC6 inhibitors
- Reactant for preparation of aminofuranopyrimidines as EGFR and Aurora A kinase inhibitors
- Reactant for preparation of inhibitors of CDK4
- Reactant for preparation of tetrahydrocarboline derivatives of as human 5-HT5A receptor ligands
- Reactant for preparation of 5-(diaminomethyl)-2,4-aminopyrimidines as dihydrofolate reductase inhibitors and antibacterial agents
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
從最近期的版本中選擇一個:
分析證明 (COA)
Lot/Batch Number
Minoru Tanaka et al.
Bioorganic & medicinal chemistry, 21(5), 1159-1165 (2013-01-23)
Indoleamine 2,3-dioxygenase (IDO) plays a significant role in several disorders such as Alzheimer's disease, age-related cataracts and tumors. A series of novel tryptoline derivatives were synthesized and evaluated for their inhibitory activity against IDO. Substituted tryptoline derivatives (11a, 11c, 11e
Takayoshi Arai et al.
The Journal of organic chemistry, 76(8), 2909-2912 (2011-03-08)
A four-step synthetic route to fully substituted chiral tetrahydro-β-carbolines (THBCs) is described. Starting from the (R,S,S)-Friedel-Crafts/Henry adduct obtained from three-component coupling of an indole, nitroalkene, and aldehyde catalyzed by imidazoline-aminophenol-CuOTf, the (1S,3S,4R)-THBCs were readily synthesized in a three-step operation including
Fang-Yuan Chang et al.
Proceedings of the National Academy of Sciences of the United States of America, 110(7), 2478-2483 (2013-01-11)
Natural product discovery by random screening of broth extracts derived from cultured bacteria often suffers from high rates of redundant isolation, making it ever more challenging to identify novel biologically interesting natural products. Here we show that homology-based screening of
Chem. Abstr., 121, 54854s-54854s (1994)
I M McDonald et al.
Journal of medicinal chemistry, 43(19), 3518-3529 (2000-09-23)
A novel series of nonpeptide CCK(2) receptor antagonists has been prepared, in which 2,7-dioxo-2,3,4,5,6,7-hexahydro-1H-benzo[h][1, 4]diazonine (5) was used as a chemical template. This uncommon ring system was obtained in a highly substituted form and in high yield by ozonolysis of
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務


![2,3,4,5-Tetrahydro-1H-pyrido[4,3-b]indole AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/376/664/07577eb6-6e8c-4237-b8c5-03da4c8e7d88/640/07577eb6-6e8c-4237-b8c5-03da4c8e7d88.png)