推薦產品
化驗
99%
mp
236-238 °C (lit.)
溶解度
1 M NaOH: soluble 50 mg/mL, clear, colorless
SMILES 字串
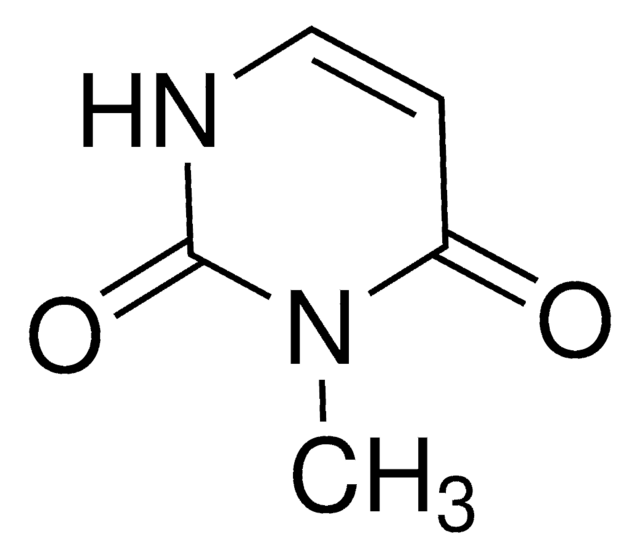
CN1C=CC(=O)NC1=O
InChI
1S/C5H6N2O2/c1-7-3-2-4(8)6-5(7)9/h2-3H,1H3,(H,6,8,9)
InChI 密鑰
XBCXJKGHPABGSD-UHFFFAOYSA-N
一般說明
1-Methyluracil is of special importance in biochemistry, since uracil attaches ribose in ribonucleic acid (RNA) just precisely at the N1 atom[1]. H-bond complex formation between 1-methyluracil and glycine has been investigated by theoretical calculations and FT-IR spectroscopy in Ar matrices[2]. It forms 1:1 complexes with 9-ethyl-8-bromo-2,6-diaminopurine and the complex structure has been determined by three-dimensional X-ray diffraction methods[3].
訊號詞
Warning
危險聲明
危險分類
Carc. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Marek Boczar et al.
The Journal of chemical physics, 128(16), 164506-164506 (2008-05-02)
Theoretical simulation of the band shape and fine structure of the N-H(D) stretching band is presented for 1-methyluracil and its deuterated derivative taking into account anharmonic coupling between the high-frequency N-H(D) stretching and the low-frequency N...O stretching vibrations, resonance interaction
Natalja Vogt et al.
The journal of physical chemistry. A, 117(44), 11374-11381 (2013-10-31)
As far as fundamental knowledge is concerned, the methyl derivatives of uracil can be considered as the simplest objects for studying the structural effects due to the substitution in the pyrimidyne nucleobases. From this point of view, 1-methyluracil is of
Parthapratim Munshi et al.
Acta crystallographica. Section A, Foundations of crystallography, 64(Pt 4), 465-475 (2008-06-19)
Anisotropic displacement parameters (ADPs) are compared for H atoms estimated using three recently described procedures, both among themselves and with neutron diffraction results. The results convincingly demonstrate that all methods are capable of giving excellent results for several benchmark systems
V I Poltev et al.
Molekuliarnaia biologiia, 29(2), 365-375 (1995-03-01)
Monte Carlo simulation of hydration of keto and enol tautomers of 9-methylguanine (G) and 1-methyluracil (U) has been performed in relation to a possible role of tautomer transitions of DNA bases in mutagenesis. The comparison of the simulation results with
V I Poltev et al.
Journal of biomolecular structure & dynamics, 9(1), 101-111 (1991-08-01)
A number of nucleic acid base pairs and complexes between the bases and the amide group of acrylamide have been studied experimentally by using mass spectrometry and theoretically by the method of atom-atom potential function calculations. It has been found
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務





![6-Methylthieno[2,3-d]pyrimidine-2,4(1H,3H)-dione](/deepweb/assets/sigmaaldrich/product/structures/393/943/c932f315-dd4b-4939-aea6-646238005e48/640/c932f315-dd4b-4939-aea6-646238005e48.png)


