推薦產品
化驗
98%
形狀
solid
bp
178-180 °C/60 mmHg (lit.)
mp
65-67 °C (lit.)
官能基
ester
SMILES 字串
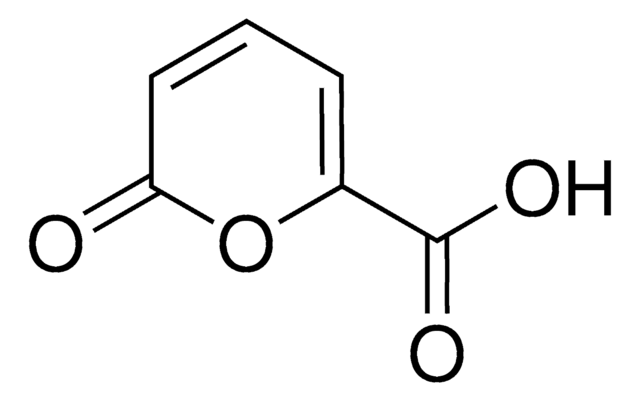
COC(=O)C1=COC(=O)C=C1
InChI
1S/C7H6O4/c1-10-7(9)5-2-3-6(8)11-4-5/h2-4H,1H3
InChI 密鑰
HHWWWZQYHPFCBY-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
Methyl coumalate is a 2-pyrone and acts as dienophile in Diels-Alder reaction. It reacts with 1,3-butadienes at 100°C to yield tetrahydrocoumarins and 4-methoxycarbonyltricyclo[3.2.1.02,7]octenes. It also reacts with cyclohexadiene to afford tetrahydronaphthalene-2-carboxylate.[1] It undergoes Diels-Alder reaction with unactivated alkenes to afford para-substituted adducts.[2]
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
從最近期的版本中選擇一個:
分析證明 (COA)
Lot/Batch Number
Aromatics from pyrones: para-substituted alkyl benzoates from alkenes, coumalic acid and methyl coumalate.
Kraus GA, et al.
Green Chemistry, 13(10), 2734-2736 (2011)
Diels-Alder reaction of methyl coumalate with 1, 3-dienes.
Imagawa T, et al.
Tetrahedron, 30(14), 2227-2231 (1974)
Suqing Zheng et al.
Organic letters, 11(17), 3978-3981 (2009-08-13)
A phosphine-catalyzed [4 + 3] annulation of modified allylic carbonates with methyl coumalate was developed. This strategy offered a powerful method for the construction of bicyclo[3.2.2]nonadiene skeleton with high stereoselectivity.
Quinolizinium Compounds by Cyclization of Pyridones from Methyl Coumalate and ?-Phenylethylamines.
Wiley RH, et al.
Journal of the American Chemical Society, 75(18), 4482-4484 (1953)
文章
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
Diels-Alder 反應是共轭二烯和烯烴(親二烯)之間形成不飽和六元環的反應。它也被稱為環化反應。
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務